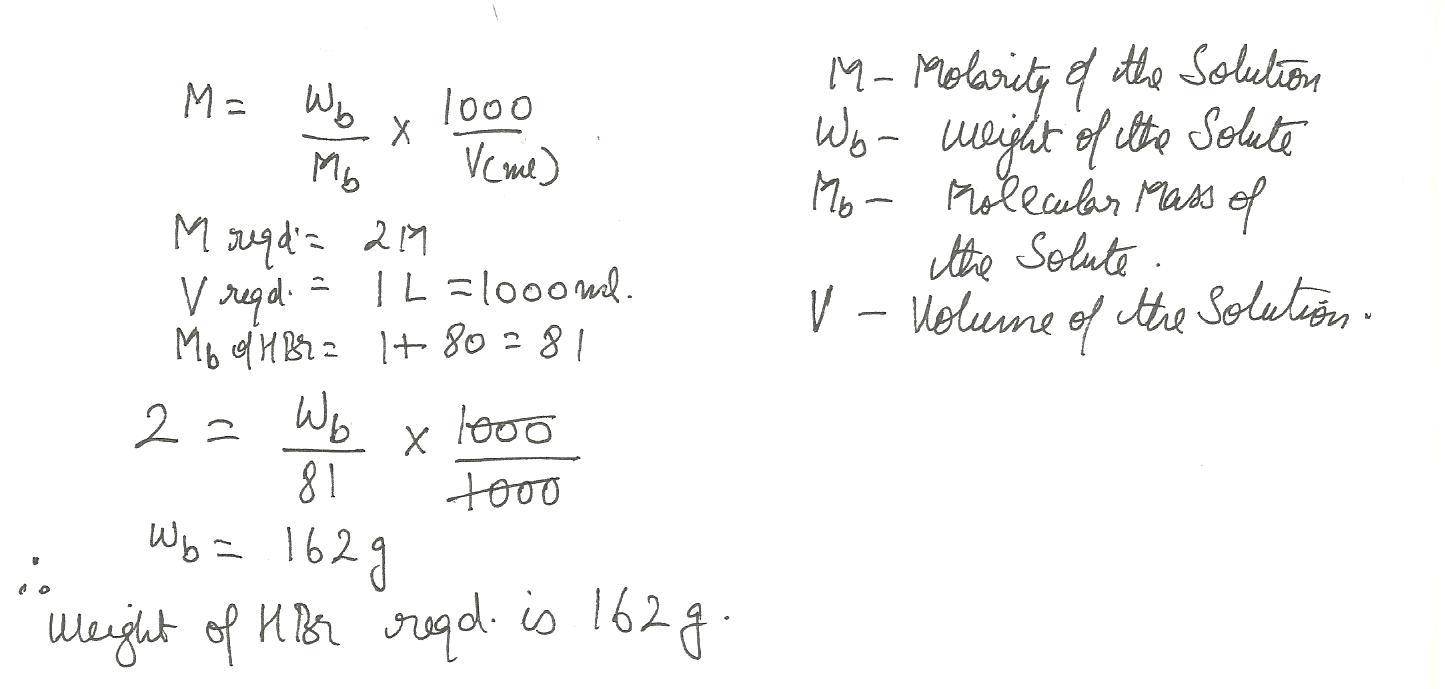Chemistry Inorganic Chemistry Level: Misc Level
An ion and its parent atom have the same
a.electron configuration
b. atomic number
c. number of charges
d. chemical reactivity
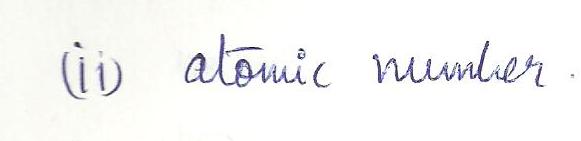
Chemistry Inorganic Chemistry Level: Misc Level
Arrange the following in order of increasing melting points.Cs, BaCI, Cdiamond , H2, HF

Chemistry Inorganic Chemistry Level: Misc Level
A mixture of Ar CH4,H2, CO, and PCL5 is released in back of the classroom. Which gas reaches the front of the classroom first?

Chemistry Inorganic Chemistry Level: Misc Level
Give the pH of a solution that is 1000 times more basic than a solution with a pH of 9.
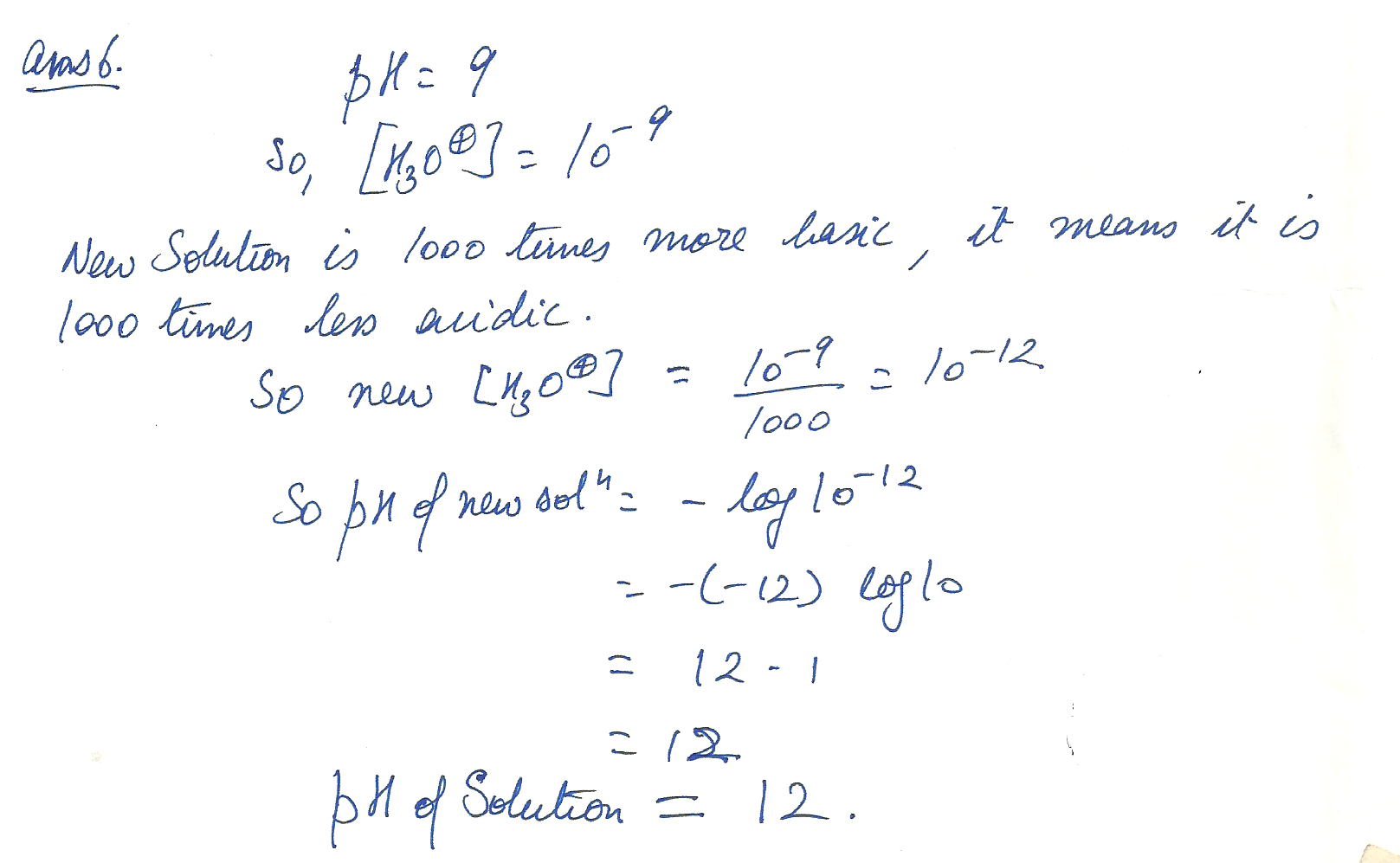
Chemistry Inorganic Chemistry Level: Misc Level
Give the pH of a solution that is 100 times less acidic than a solution with a pH of 3.
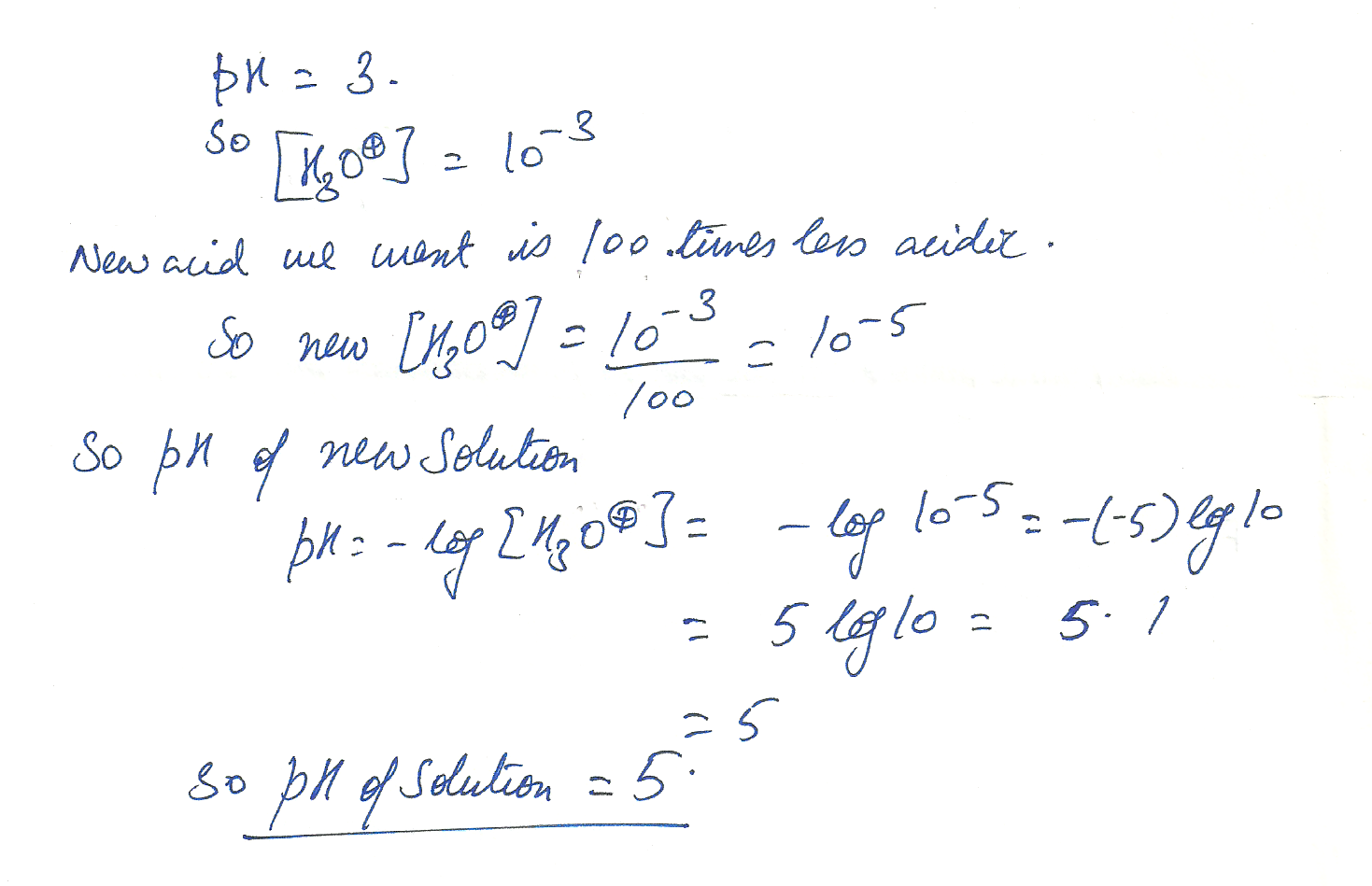
Chemistry Inorganic Chemistry Level: Misc Level
Identify the acid, base, conjugate acid, and conjugate base in the following H2S +NH3 <-------> NH4 +HS-
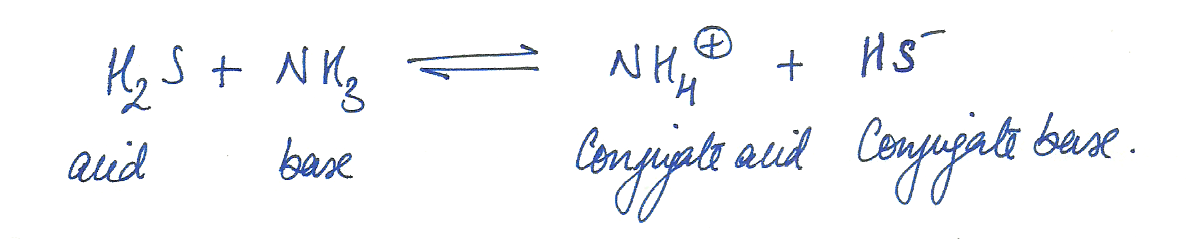
Chemistry Inorganic Chemistry Level: Misc Level
Write the chemical reaction that describes the reaction of the weak acid HNO2 with water. Identify the acid, base, conjugate acid, and conjugate base in your reaction.

Chemistry Inorganic Chemistry Level: Misc Level
Give the pH, (OH- ) and indicate acid, base, or neutral for a solution that has (H3O+) of 1x10-11. ( 10 with a little minus sign and little 11 to the upper right of the 10. )

Chemistry Inorganic Chemistry Level: Misc Level
What Volume of 0.200 M HCI solution is needed to exactly neutralize 25.0 ML of 0.150 M NaOH solution?
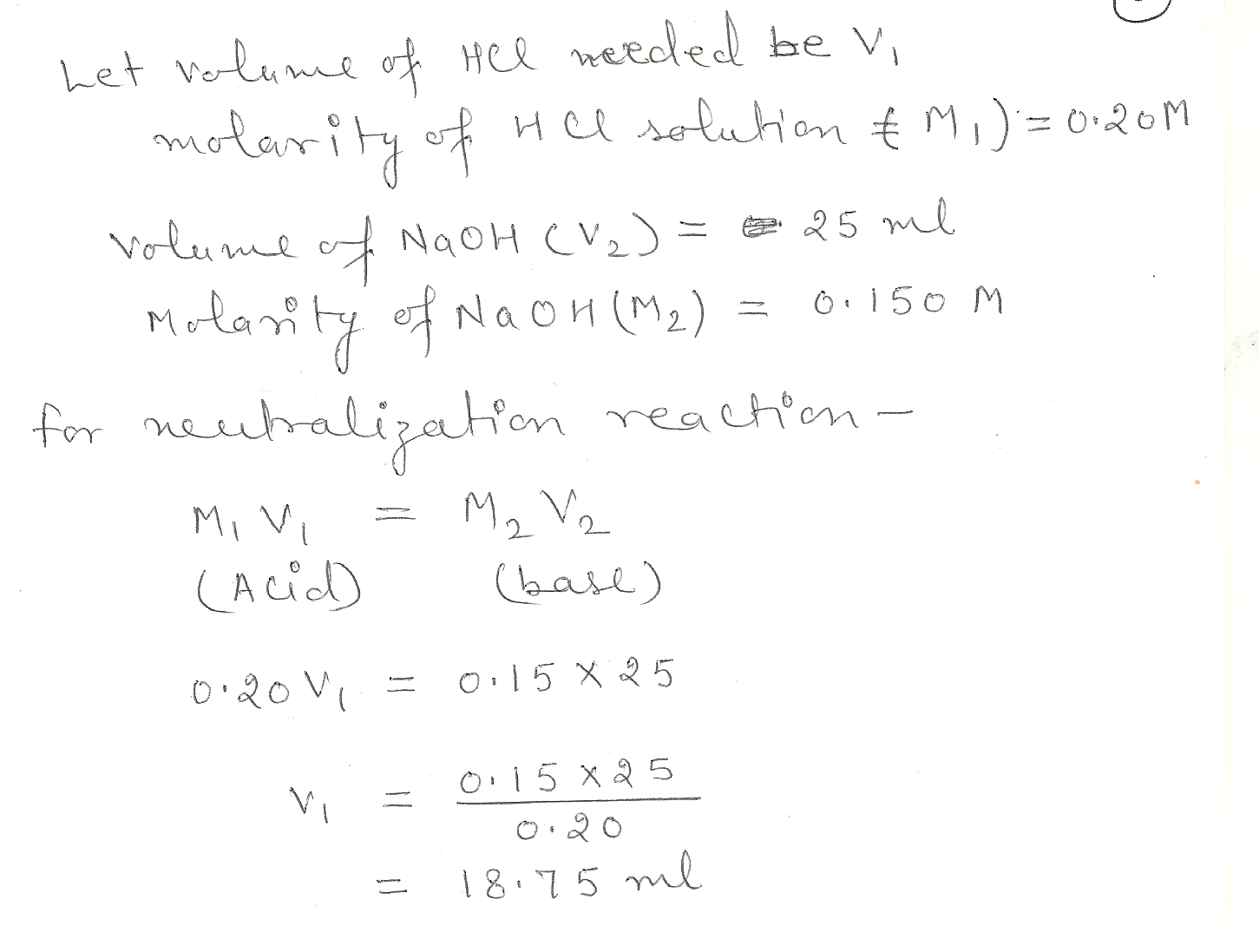
Chemistry Inorganic Chemistry Level: Misc Level
Calculate the new concentraion (molarity) that results when 250. ML of water is added to 125 MLof 0.251 M HCI
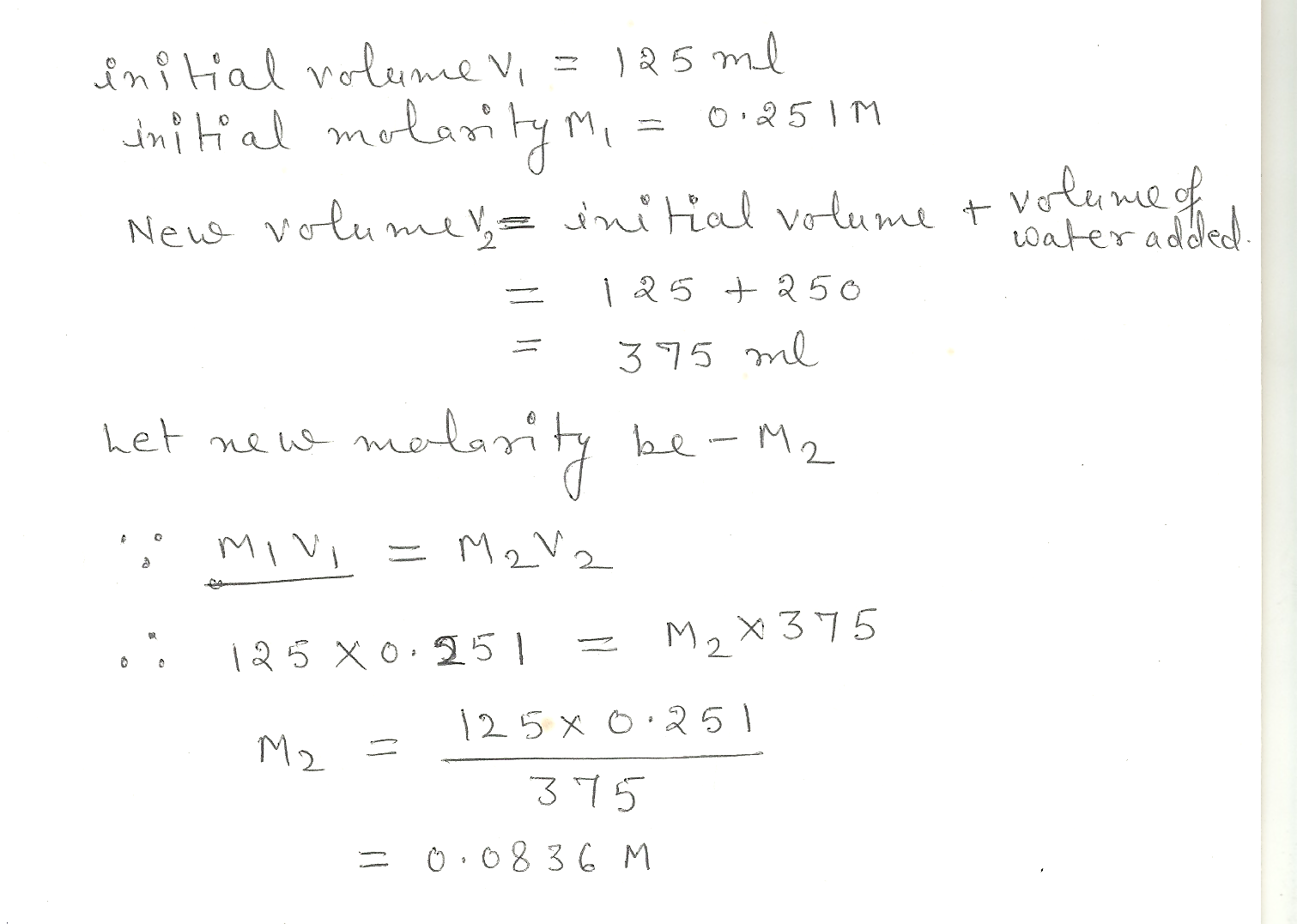
Chemistry Inorganic Chemistry Level: Misc Level
Calculate the number of moles and number of grams of HCI contained in 1.29 L of 0.411 M H CI
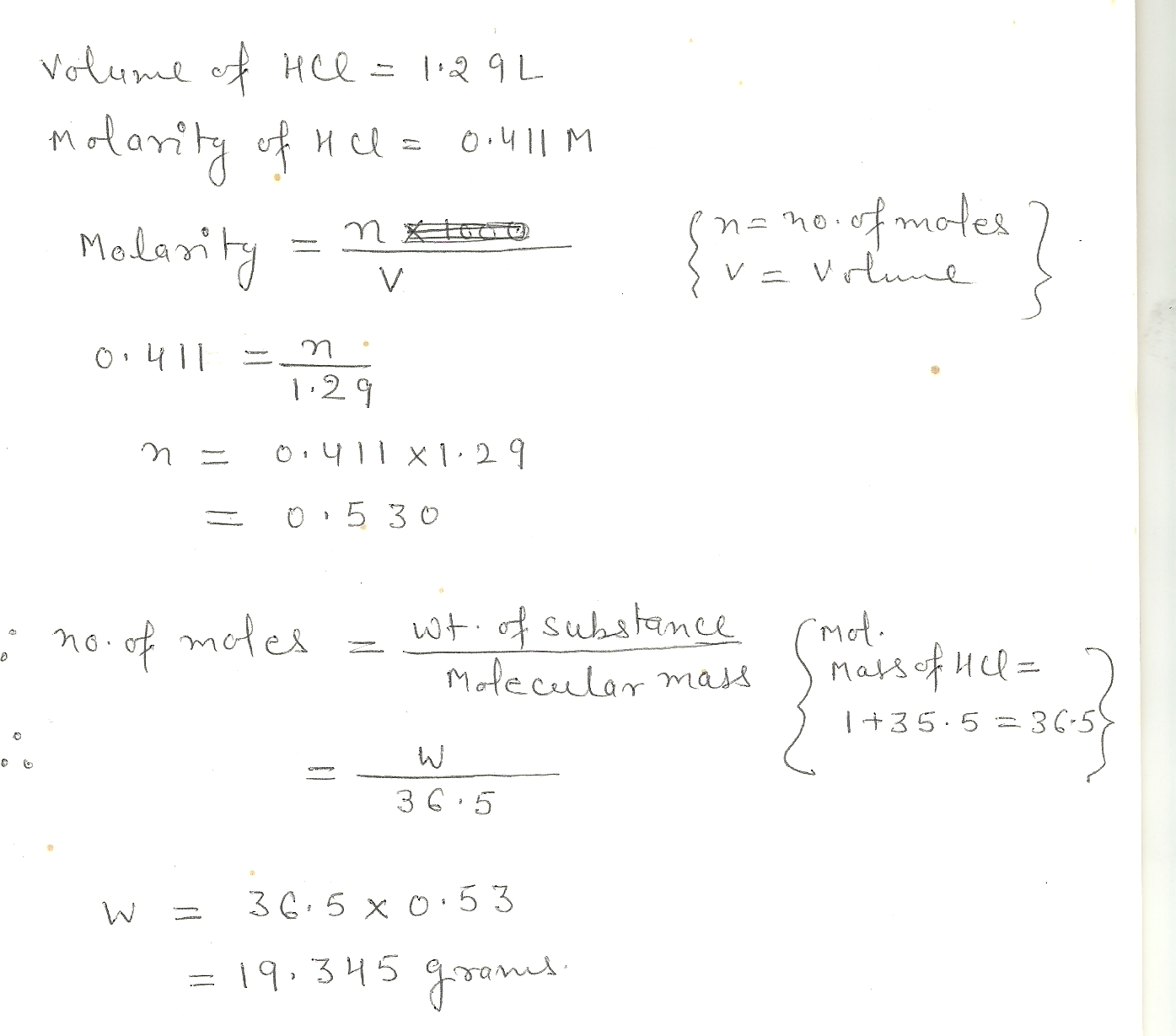
Chemistry Inorganic Chemistry Level: Misc Level
How many grams of sugar are contained in 250. g of an aqueous mixture that contains (by mass) 10.% sugar and 5.0% alcohol. ?
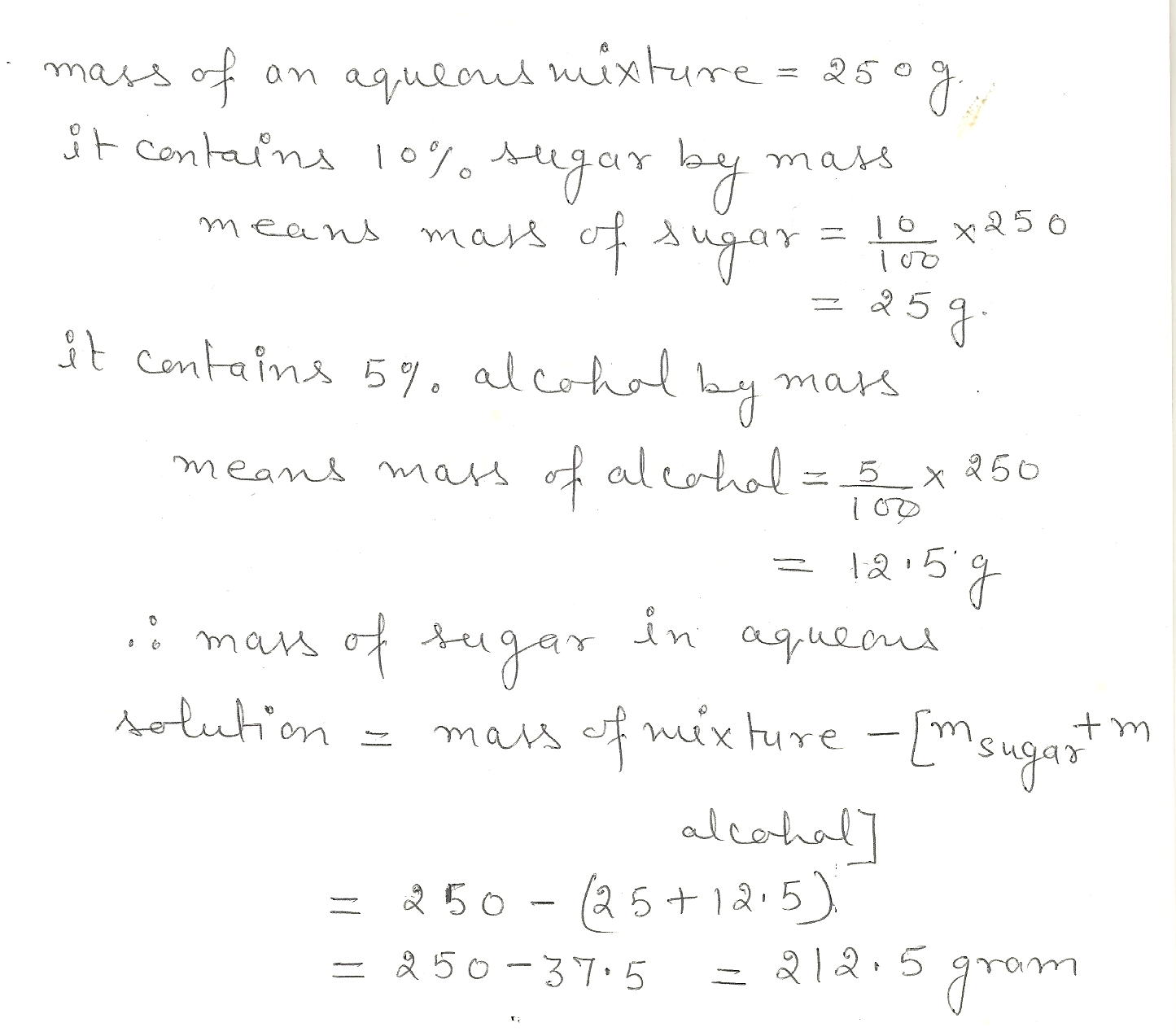
Chemistry Inorganic Chemistry Level: Misc Level
Potassium permanganate , KMn04, is produced commercially by oxidising aqueous potassium manganate, K2MnO4, with chlorine gas.The chemical equation is:
2MnO42-(aq)+ CI2(g) +2K+ (aq) -> 2KMn04 (s) +2CI- (aq)
What volume of CI2 (g), measured at STP, is needed to produce 10.0 g of KMn04?
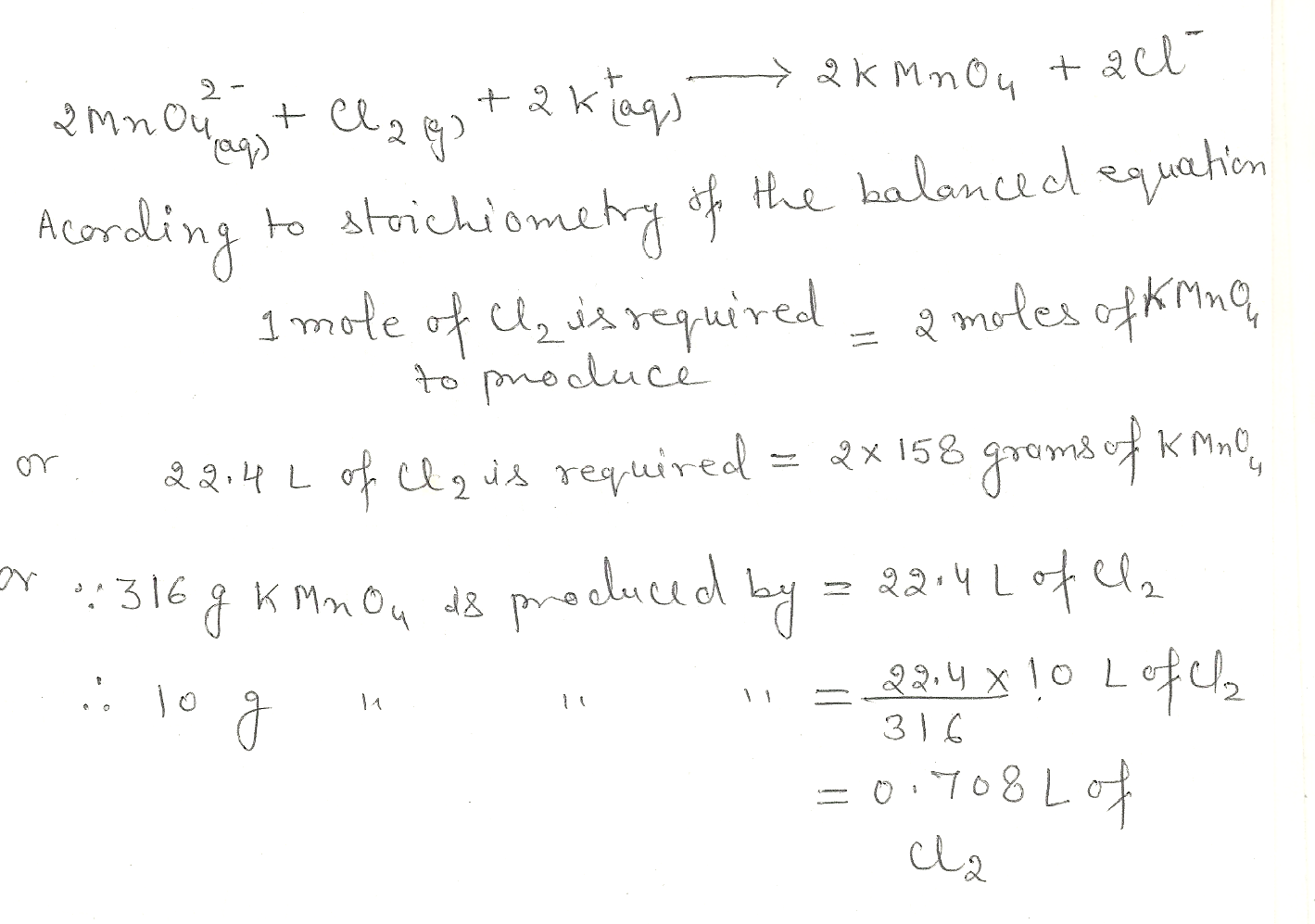
Chemistry Inorganic Chemistry Level: Misc Level
(b) what is the pH for the following solution? 0.001 M HNO3 ch.9

Chemistry Inorganic Chemistry Level: Misc Level
How many grams of HBr must be dissolved to make 1.0 L of 2.0 M HBr solution?