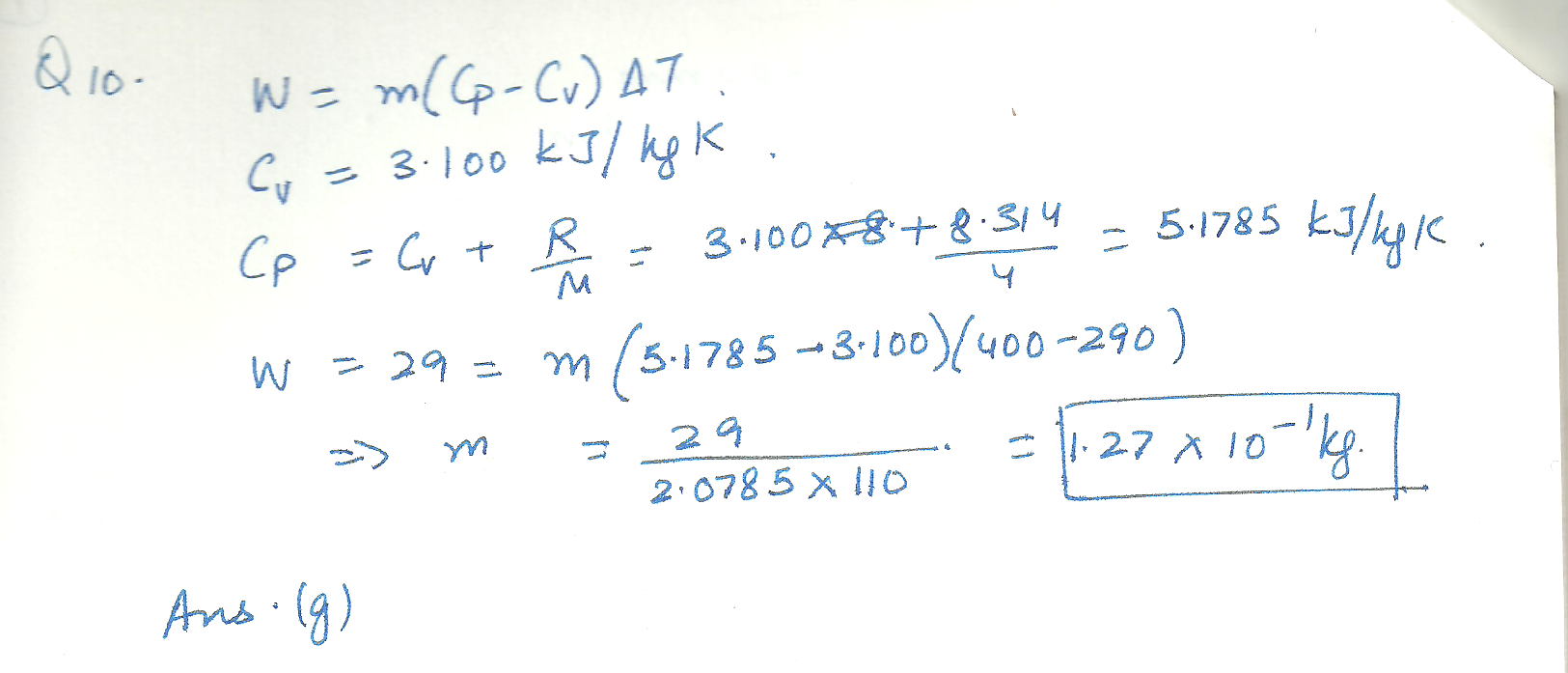Physics Heat & Thermodynamics Level: Misc Level
Answer the following question(s), showing your calculations. An 8.00x 102-g block of lead is heated in boiling water, 100.0%C, until the block, s temperature is the same as the water,s The lead is then removed from the boiling water and dropped into 2.50x 10 2 g of cool water at 12.2 % C. After a short time, the
a. How much heat is gained by the cool water?
b. On the basis of these measurements, what is the specific heat of lead?

Physics Heat & Thermodynamics Level: Misc Level
Answer the following question(s), showing your calculations. An 8.00x 102-g block of lead is heated in boiling water, 100.0%C, until the block, s temperature is the same as the water,s The lead is then removed from the boiling water and dropped into 2.50x 10 2 g of cool water at 12.2 % C. After a short time, the
a. How much heat is gained by the cool water?
b. On the basis of these measurements, what is the specific heat of lead?

Physics Heat & Thermodynamics Level: Misc Level
Given that ice has a heat of fusion of 3.34x 10 5. J/kg, how much heat must be added to 2.3 kg of ice at 0.0 % C to liquid water at 0.0 %C?
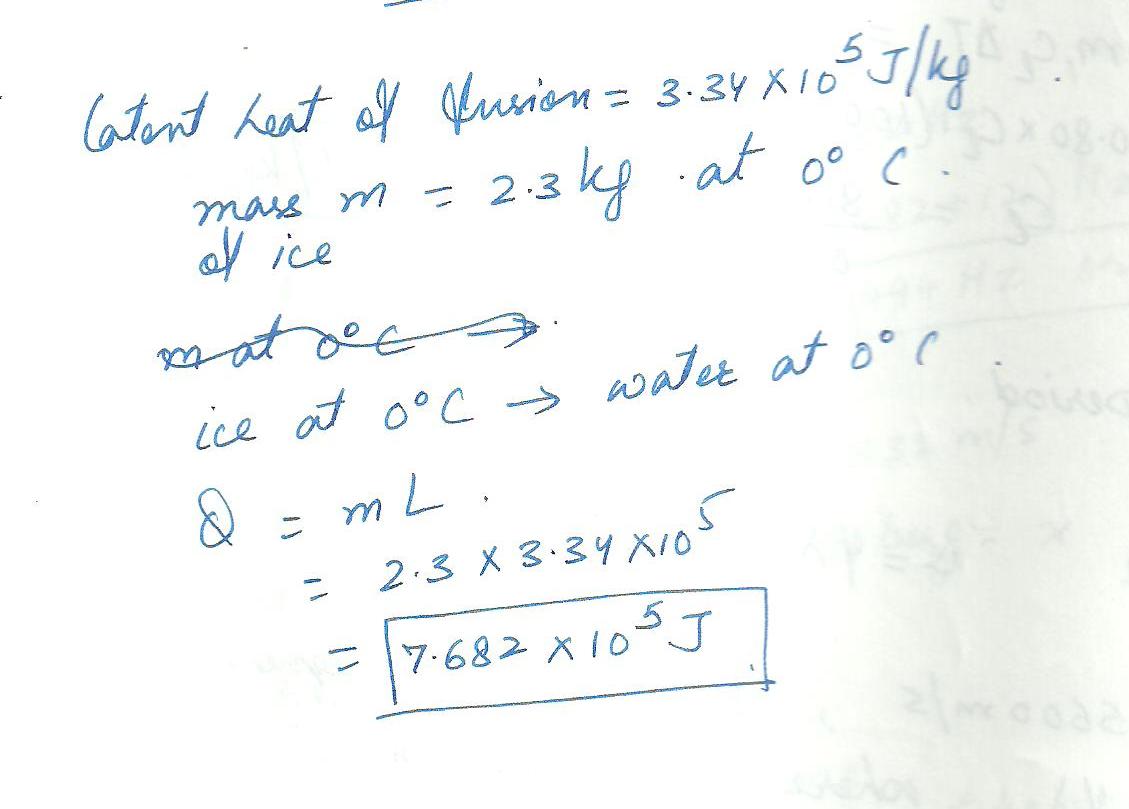
Physics Heat & Thermodynamics Level: Misc Level
The specific heat of methanol is 2450 J/kg-K.Calculate the amount of heat that must be added to 0.21 kg of methanol to raise its temperature by 25% C.
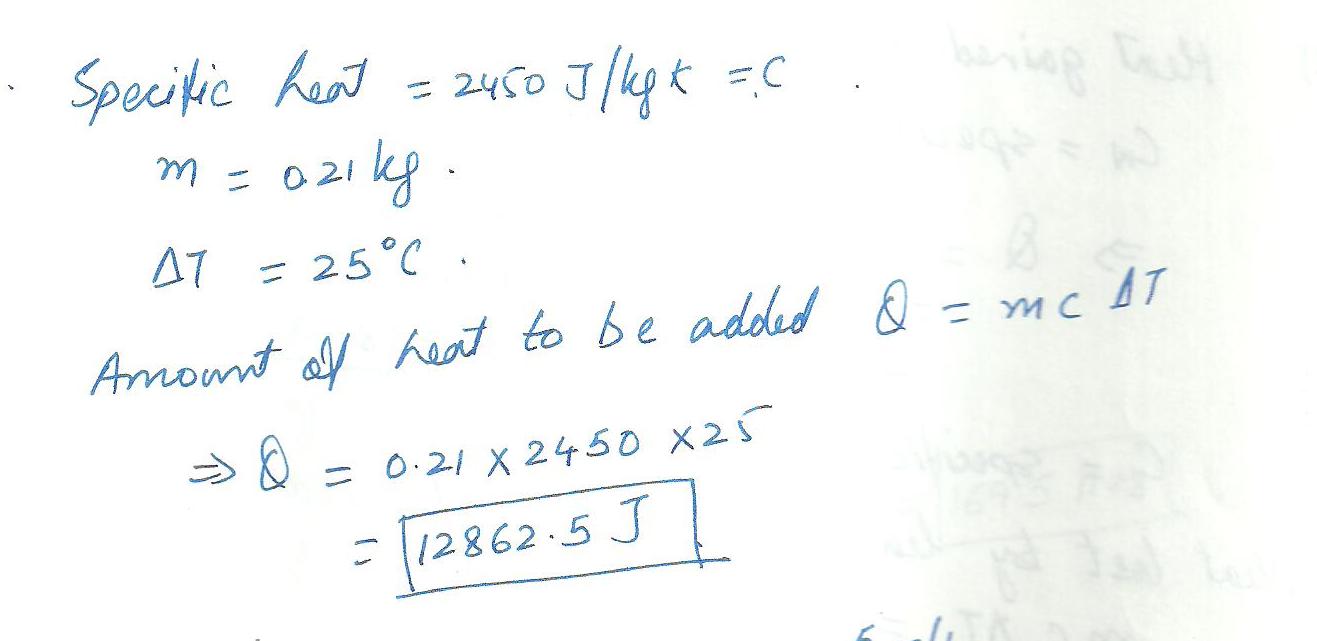
Physics Heat & Thermodynamics Level: Misc Level
Entropy is the measure of a system,s
[A] disorder [B] temperature[C] thermal equilibrium [D] thermal energy

Physics Heat & Thermodynamics Level: Misc Level
The amount of heat needed to melt 1 kg of a substance is the --[A] heat of fusion [B] entropy [C] specific heat [D] heat of vaporization
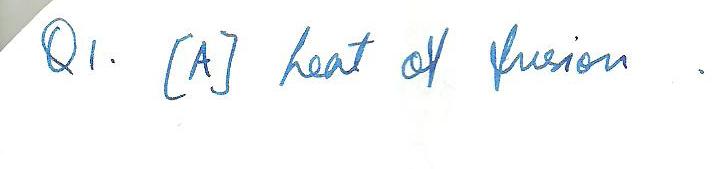
Physics Heat & Thermodynamics Level: Misc Level
The Concorde is 62 m long when its temperature is 23o C. In flight, the outer skin of this supersonic aircraft can reach 115o C due to air friction. The coefficent of linear expansion of the skin is 1.8 x 10-5 /oC. Find the amount by which the Concorde expands.
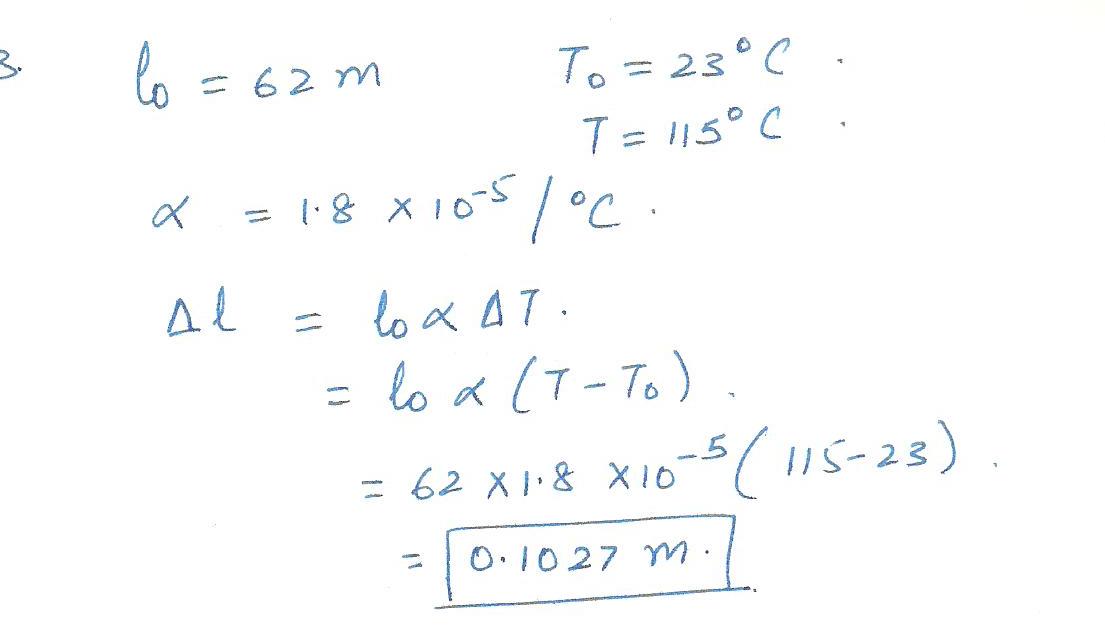
Physics Heat & Thermodynamics Level: Misc Level
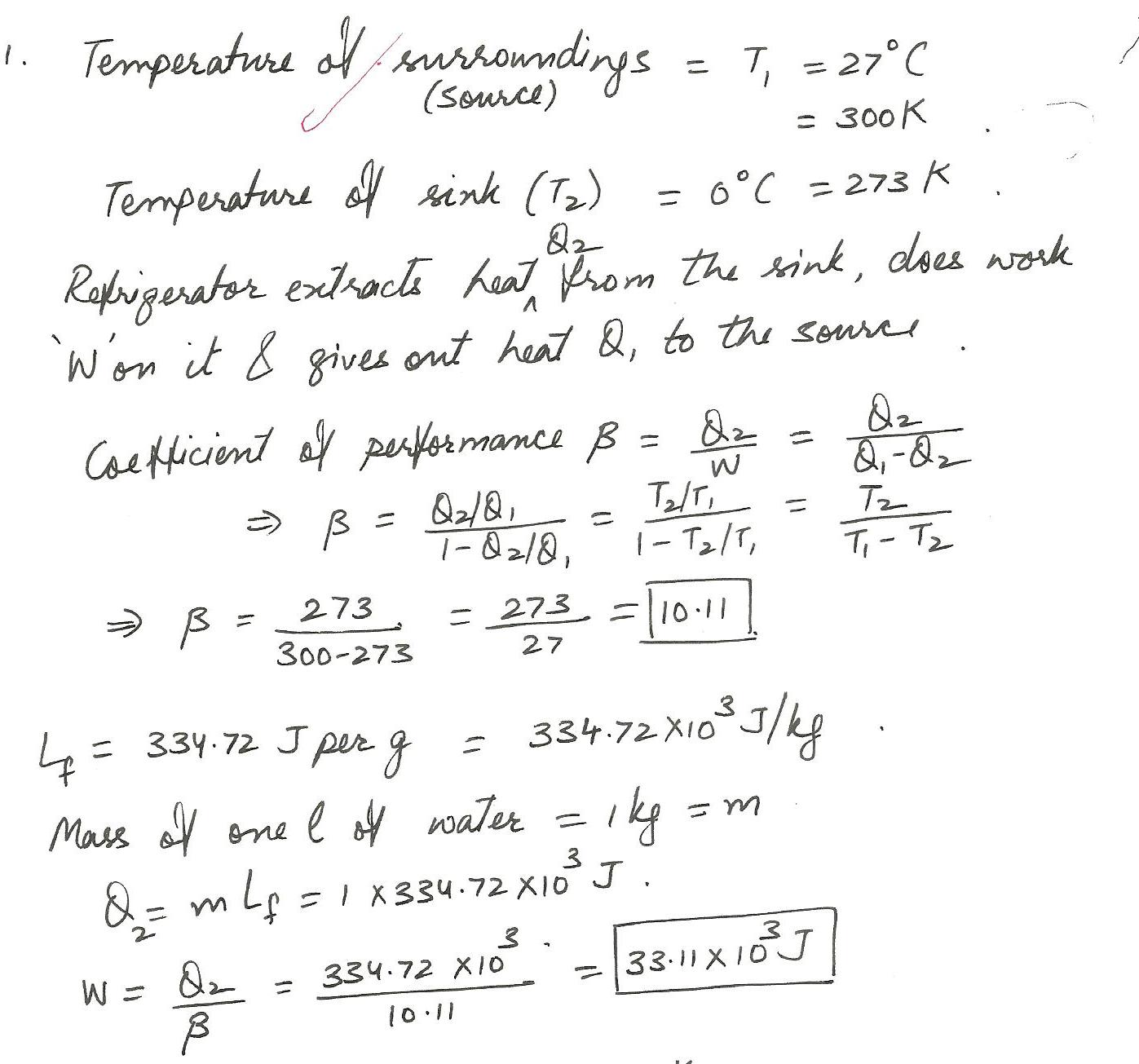
A refrigerator opertes in a cyclic and reversible manner between 0 and 27o C.How much work must be done in freezing 1 L of water ?Calculate the coefficient of performance given that the latent heat of fusion of water is 334.72 J/g.
Physics Heat & Thermodynamics Level: Misc Level
A perfectly insulated calorimeter contains 500 mL of water at 30 degrees Celsius and 45 g of ice at 0 ( zero ) degrees celsius. Determine the final temperature of the system.Keep units consistant.
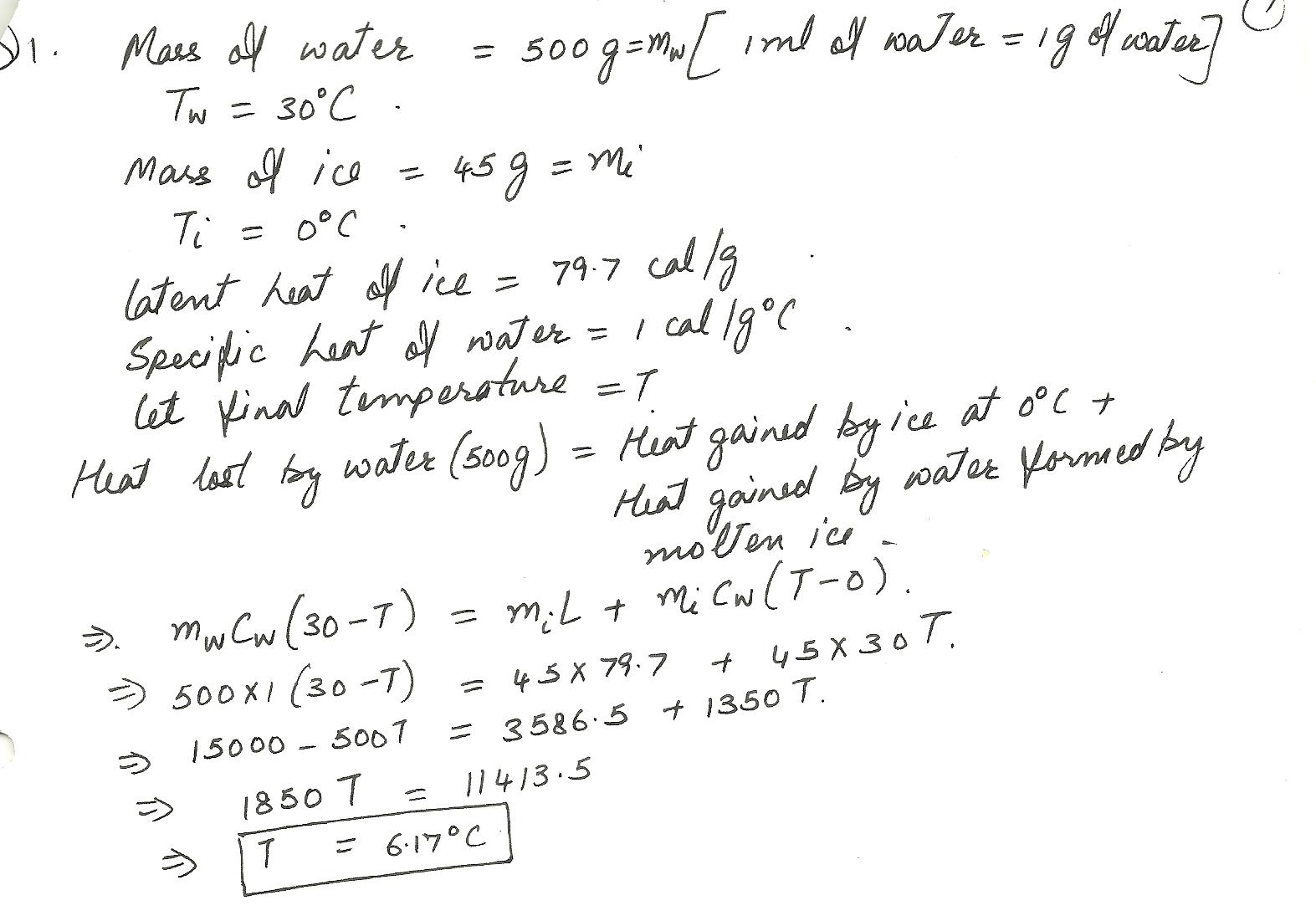
Physics Heat & Thermodynamics Level: Misc Level
Aluminum has a positive coefficient of thermal expansion. Consider a round hole that has been drilled in a large sheet of Aluminum. As the temperature increases and the surrounding metal expands, the hole diameter will:
(a) decrease
(b) increase
(c) depends how much metal surrounds the hole
(d) remain constant
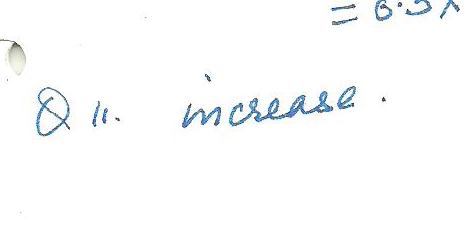
Physics Heat & Thermodynamics Level: Misc Level
How much heat is added 500 g of water to raise its temperature from 15 %C to 85 % C?
(a) 35 ]
(b) 3.5 x10 4 ]
(c) 1.5 x10 5]
(d) 1.5 x10 8]
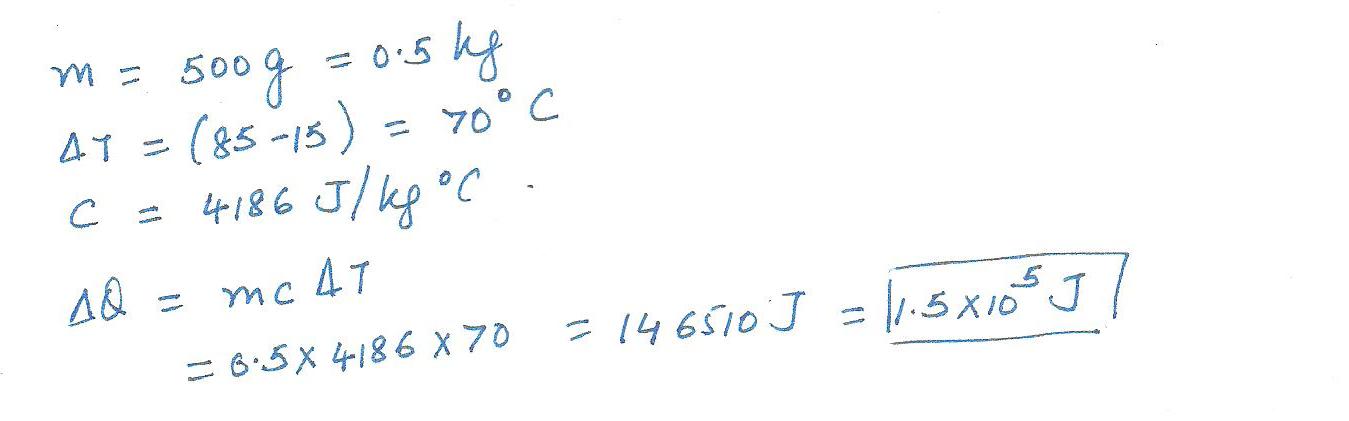
Physics Heat & Thermodynamics Level: Misc Level
Both the pressure and volume of a given sample of an ideal gas double. This means that its temperature in Kelvins must
(a) quadruple.
(b) reduce to one- fourth its original value.
(c) reduce to one -helf its original value.
(d) remain unchanged.
(e) double.
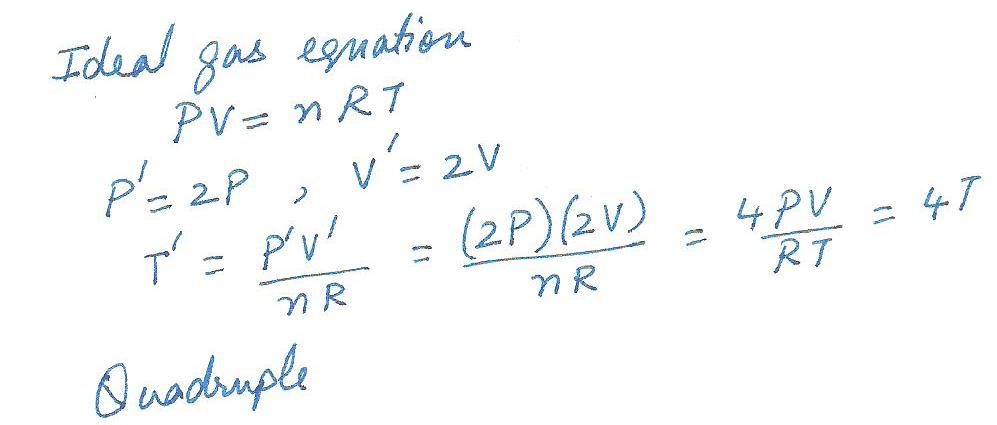
Physics Heat & Thermodynamics Level: Misc Level
a metal wire is the thermal contact with two heat reservoirs at both of its ends. Reservior 1 is at a temp of 694 k, and reservior 2 is at a temp of 263 k. what is the total change in entropy (in J/K) arising from the conduction of 1841 J of heat through the wire.
a.) 4.347 b.) 5.434 c.) 6.793 d.) 8.491 e.) 10.613 f.) 13.267 g.) 16.583 h.) 20.729
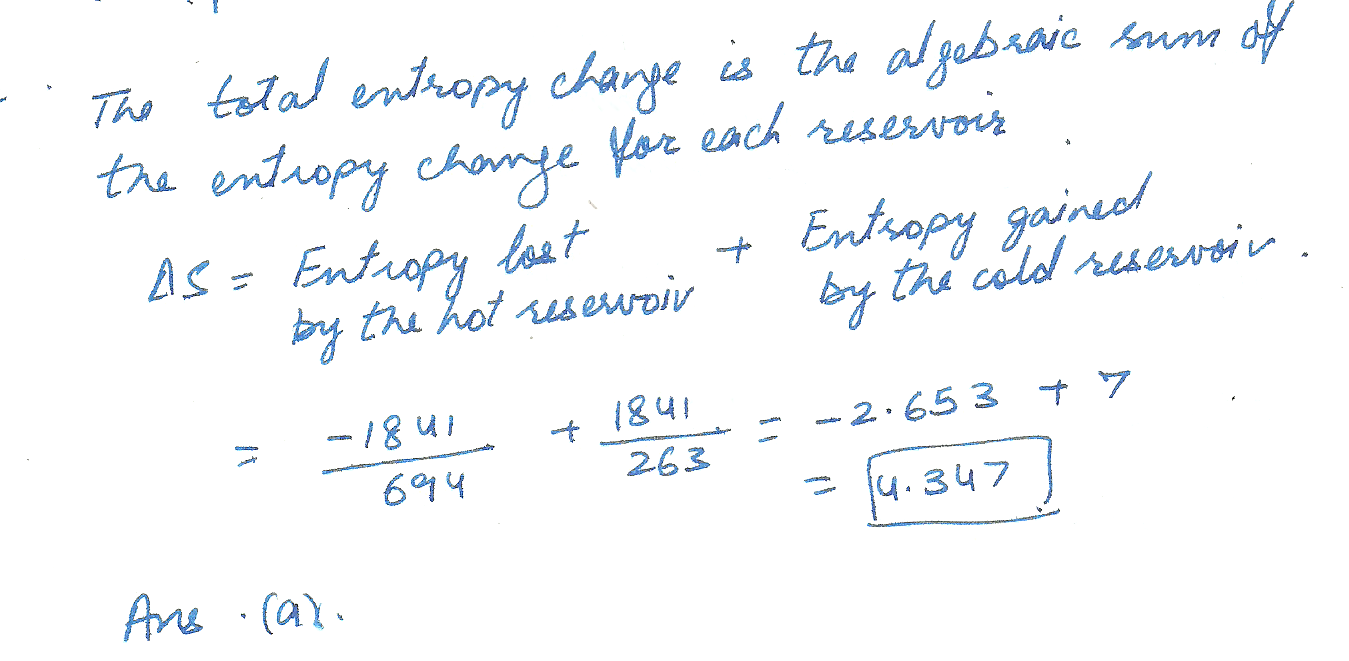
Physics Heat & Thermodynamics Level: Misc Level
a gas is compressed at a constant pressure of 0.831 atm from 10.01 L to 7.71 L. During the process, 440 J of energy leaves the gas by heat. What is the change in the internal energy of the gas /(in J)
a.) -134 b.) -151 c.) -171 d.) -193 e.) -218 f.) -246 g.) -278 h.) -315
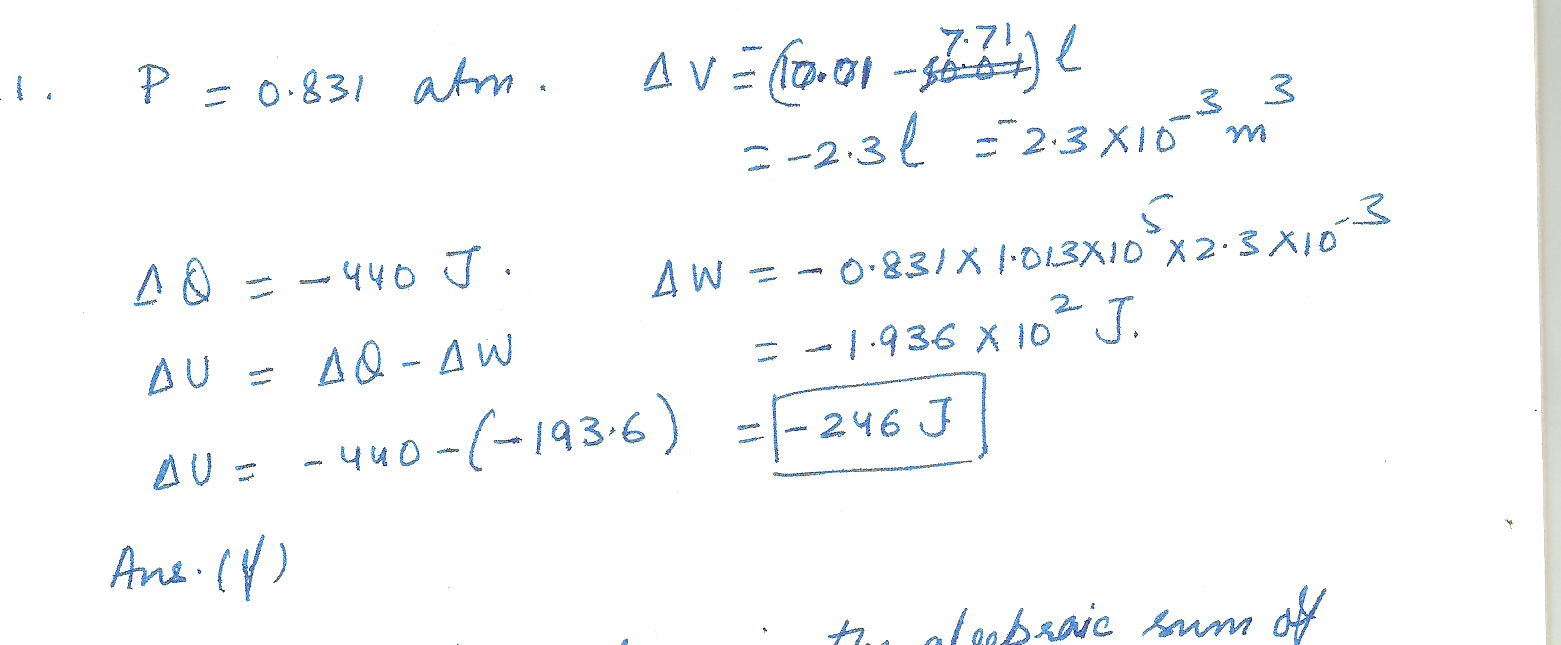
Physics Heat & Thermodynamics Level: Misc Level
a sample of helium -4 behaves as an ideal gas as it is heated at constant pressure from 290 k to 400 k, If 29 J of work is done by the gas during this process, what is the mass of helium present?
a.) 2.29*10^-2 b.) 3.05*10^-2 c.) 4.06*10^-2 d.) 5.39*10^-2 e.) 7.17*10^-2 f.) 9.54*10^-2 g.) 1.27*10^-1 h.) 1.69*10^-1