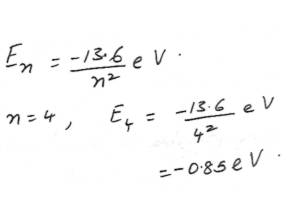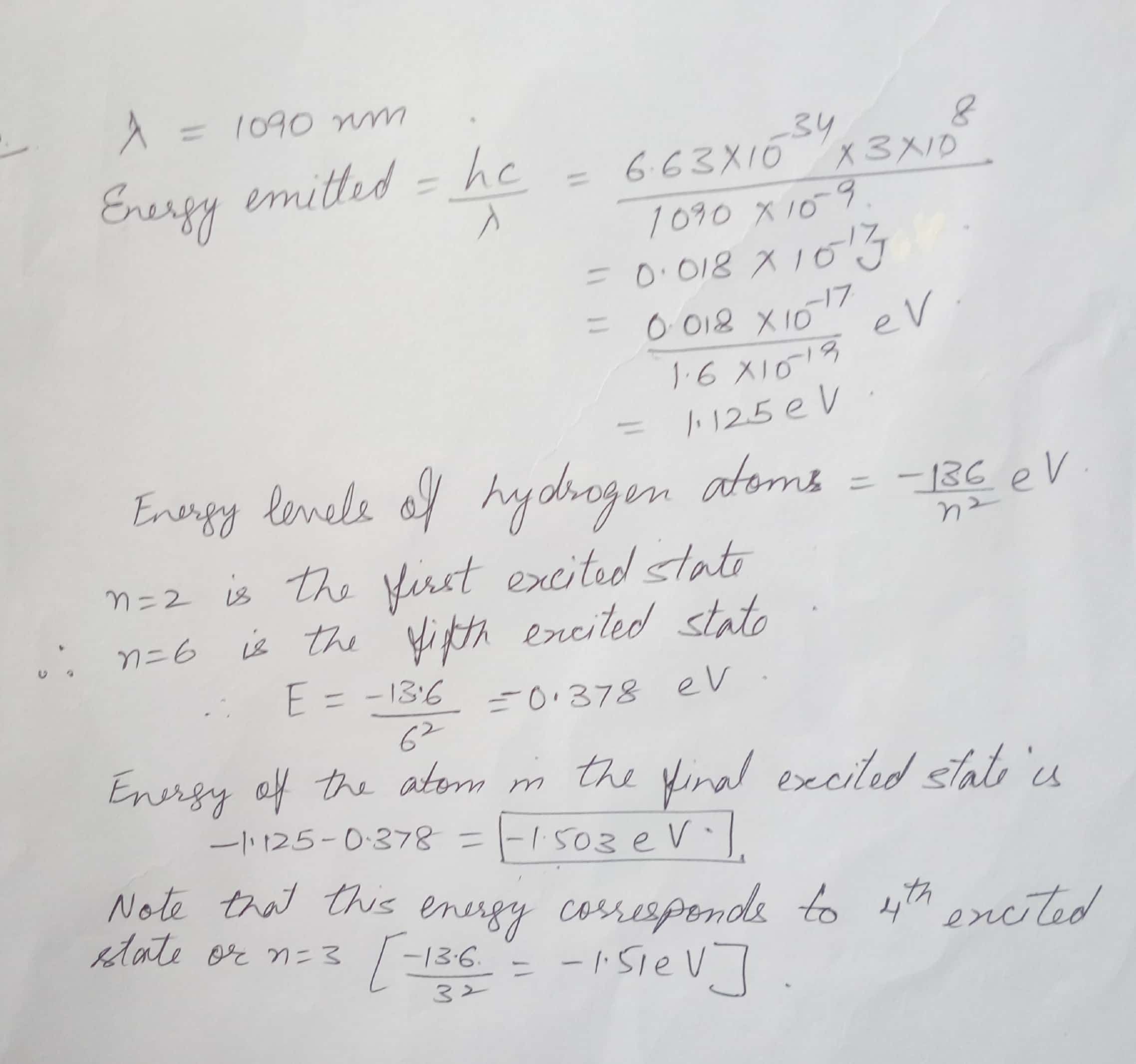Class 12 Physics - Atoms
Physics Modern Physics Level: Misc Level
Hydrogen atoms are usually found in the ground state, that is, the electron is in the n= 1 level . what wavelength light striking the atom will excite it to the n= 2 level?
Physics Modern Physics Level: University
AtomFind the energy for a hydrogen atom in the stationary state n=4.
Physics Modern Physics Level: High School
Bohr’s Atomic Model
Hydrogen emits a photon with wavelength lamda = 435 nm . Which transition between energy levels does this correspond to ?Physics Modern Physics Level: High School
Bohr’s Atomic Model
What is the energy difference between an electron in n = 3 state and n = 5 state in hydrogen ? If the electron makes a transition from n = 5 to n = 3 this energy difference is carried away by a single photon . Calculate the wavelength expressed in nm , and the frequency of this emitted photon . In what part of the electromagnetic spectrum does it lie ?Physics Modern Physics Level: High School
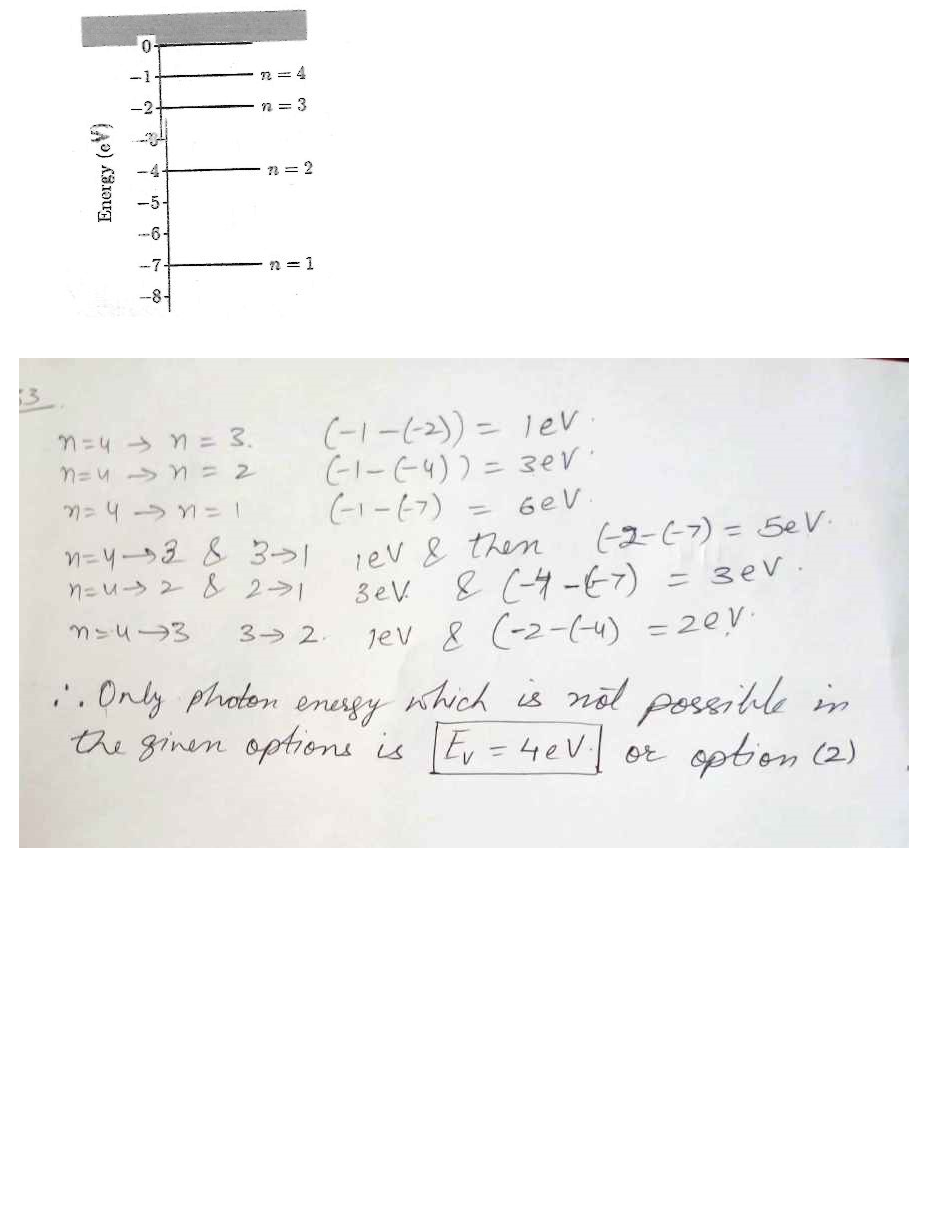
A hypothetical atom has four energy states as shown in the figure at “click here" Which of the following photon energies Ev could not be found in the emission spectra of this atom after it has been excited to the n = 4 state ?
1) Ev = 5 eV
2) Ev = 4 eV
3) Ev = 1 eV
4) Ev = 3 eV
5) Ev = 2 eV
Physics Modern Physics Level: High School
Bohr’s Atomic Model
The electron of a hydrogen atom is initially at the orbit n = 4 , If this electron first goes to the orbit n = 3 and then to the orbit n = 2 and then the orbit n =1 , how many photons are emitted during these transitions ? How many photons are emitted if the electron directly falls from the orbit n = 4 to the orbit n = 1 ?Physics Modern Physics Level: High School
Bohr's Atomic Model
A hydrogen atom is in the fifth excited state when it emits a photon with a wavelength of 1090 nm . Note than n = 2 is the first excited state . Calculate the energy of the atom in the final state .
Physics Modern Physics Level: High School
Atom
The highest energy level in an atom is itsa) ionization level
b) emission level
c) absorption level
d) photon level
e) ground state
Physics Modern Physics Level: High School
Atom
An excited atom gains energy by ?a) emitting a photon
b) absorbing a photon
c) undergoing a photoelectric effect
d) increasing its de Broglie wavelength
e) the uncertainty principle