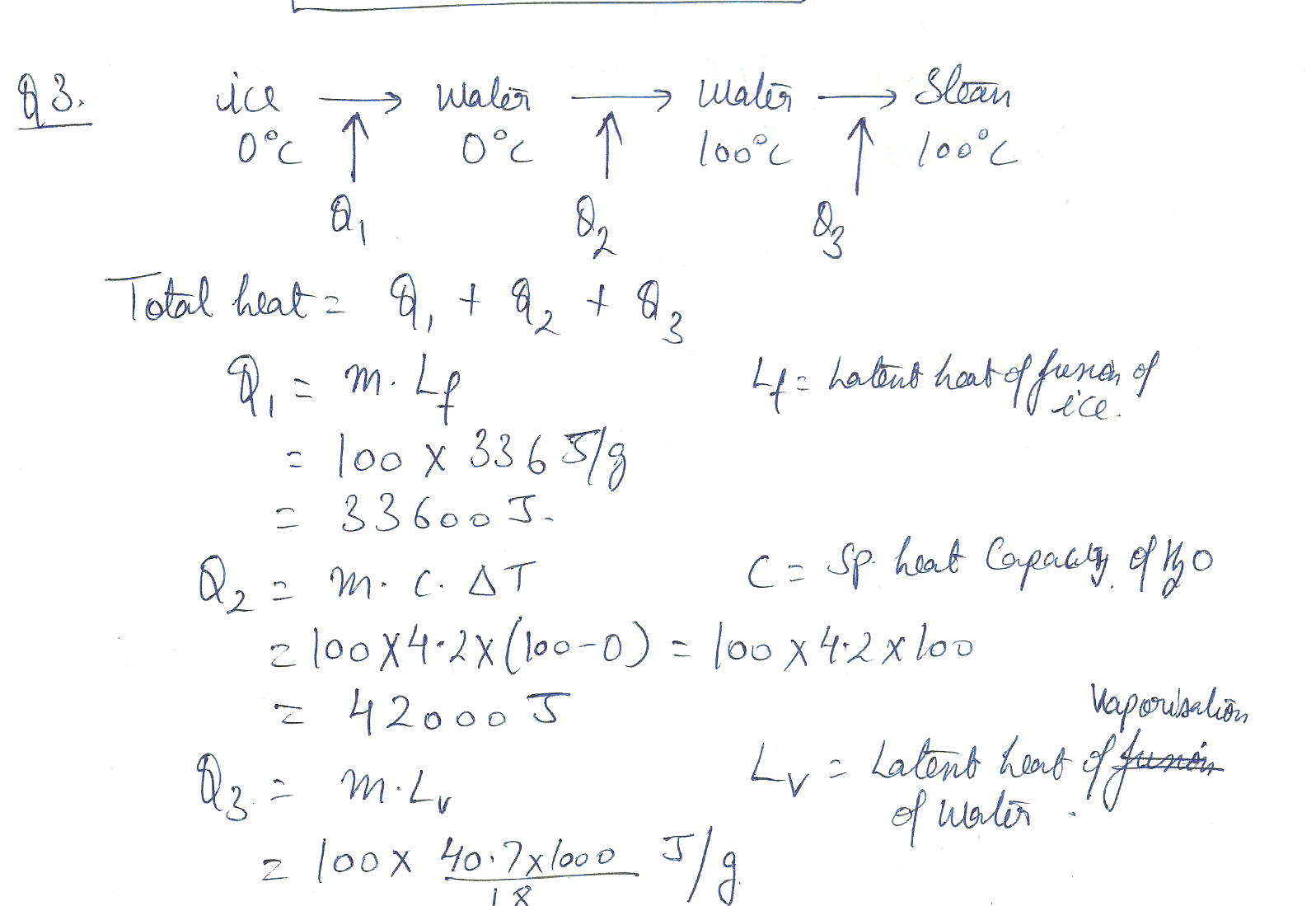4816
Chemistry Mole Concept Level: Misc Level
Calculate the total heat required to melt 100 g of ice at 0 degrees C, heat it to 100 degrees C, and then vaporize it at that temperature. (water std enthalpy of vap = 40.7 kJ /mol)
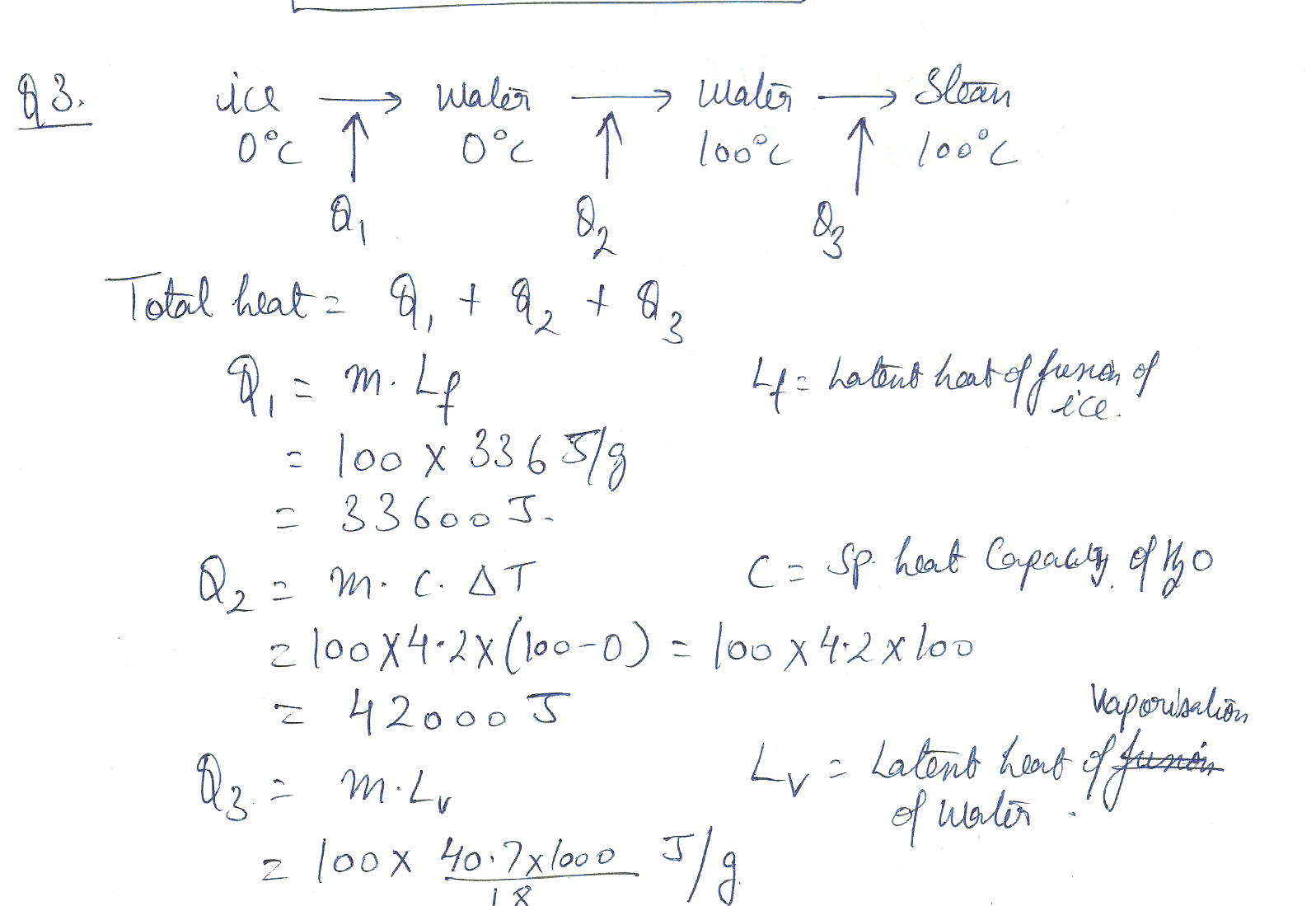
Chemistry Mole Concept Level: Misc Level
Calculate the total heat required to melt 100 g of ice at 0 degrees C, heat it to 100 degrees C, and then vaporize it at that temperature. (water std enthalpy of vap = 40.7 kJ /mol)