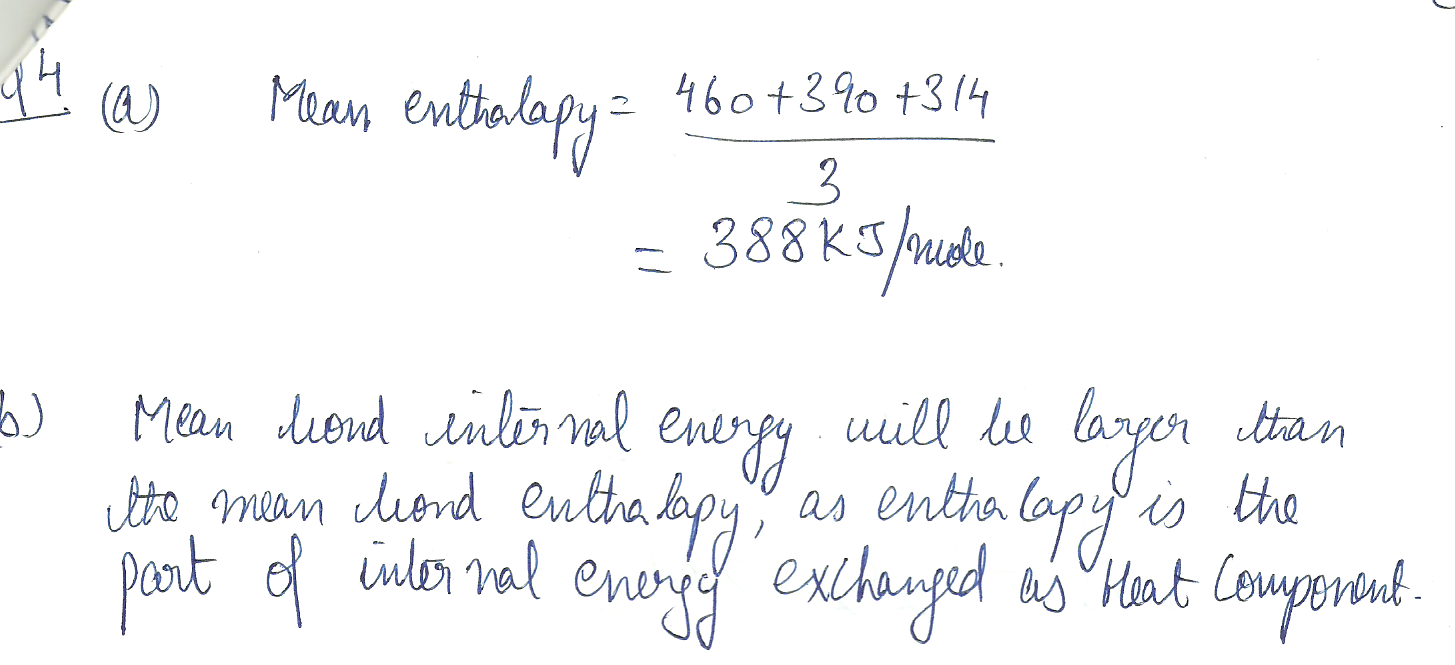4817
Chemistry Physical Chemistry Level: Misc Level
The enthalpy changes accompanying the dissociation of succesive bonds in N H 3(g) are 460, 390, and 314 kJ /mol respectively. a) What is the mean enthalpy of an N-H bond?
b) Do you expect the mean bond internal energy to be larger or smaller then the mean bond enthalpy?