Chemistry Physical Chemistry Level: Misc Level
Which of the following is NOT a property of an lonic compound?
a. low boiling point
b. brittleness
c. hardness
d. molten compound conducts electricity
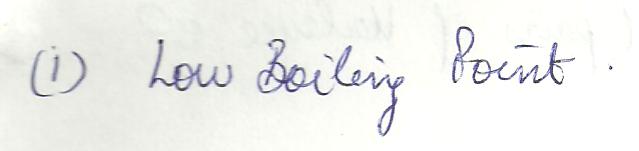
Chemistry Physical Chemistry Level: Misc Level
What volume of physiological saline (0.900% w/v NaCI) can be prepared from 90.0 mI of 2.75% w/v NaCI solution?

Chemistry Physical Chemistry Level: Misc Level
Write the eaustions of Ca (OH)2 and describe why this is more soluble in acid than water

Chemistry Physical Chemistry Level: Misc Level
The Ksp of PbBr2 is 8.9 x 10-6 At mass Pb 207 g: Br 80g
a) What is concentration of pb+2 in solution?
b) What is the concentration of Br-in solution?
c) What is the molar solubility of pbBr2 in water?

Chemistry Physical Chemistry Level: Misc Level
Write the equations for the following titrations. Using the choices (acidic, neutral, basic) describe the solutions after each titration
a) a strong acid with a strong base
b) a weak acid with a strong base
c) a weak base with a strong acid

Chemistry Physical Chemistry Level: Misc Level
Calculate the ration of conjugate base to acid in order to prepare a buffer with a p H of 10.
Ka for the acid is 4.2 x 10-7
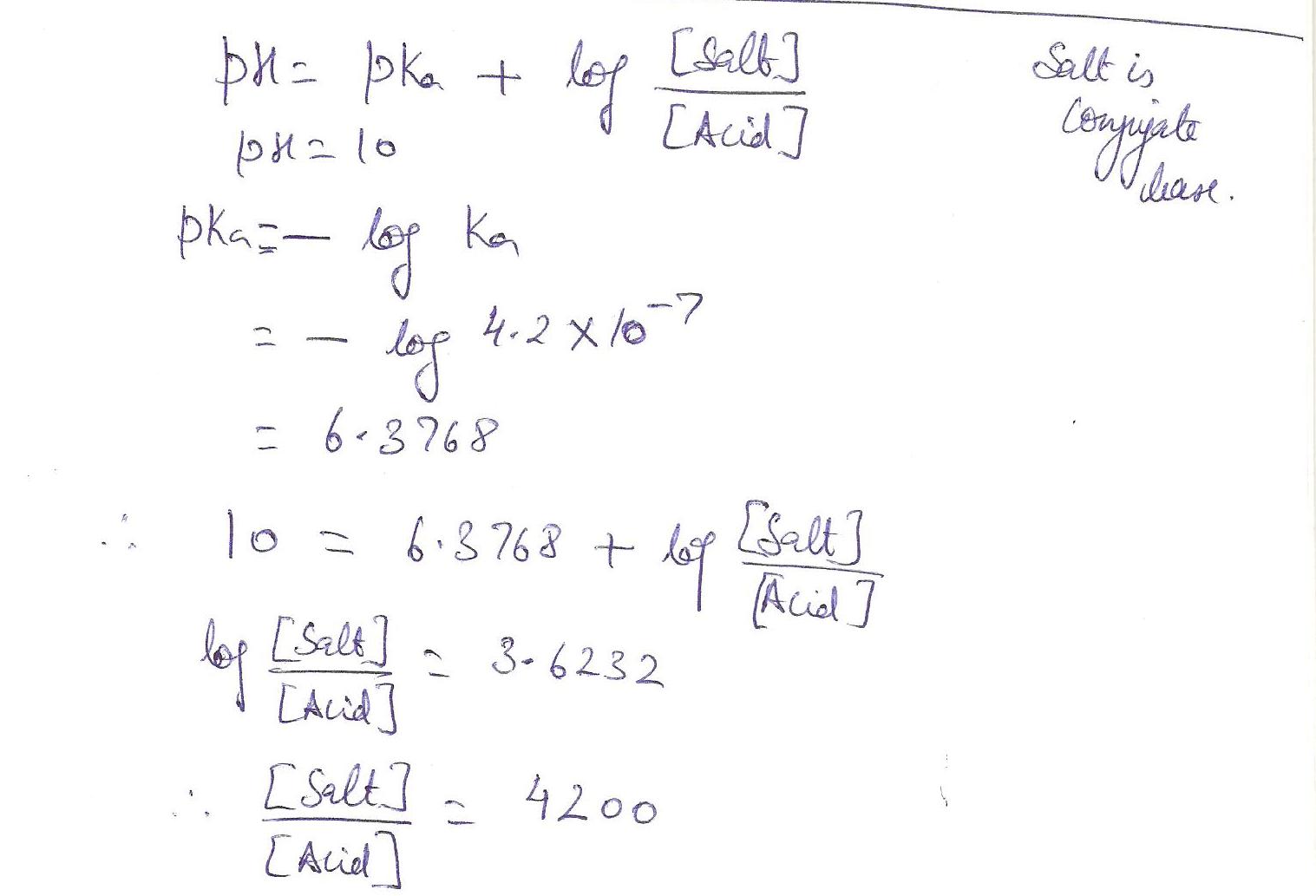
Chemistry Physical Chemistry Level: Misc Level
Using the Clausius Clapeyron determine the vapor pressure of water at 50 degree celcuis. The molar heat of evaporation of water is 40.7 kJ/mol

Chemistry Physical Chemistry Level: Misc Level
How much heat is released when 40 g of steam at 250 degrees celcisus cools down and condenses to water at 30 degrees celcius? (Specific Heat of H2O (I)4. 18 J/g degree celcius, Specific Heat of H20 (g)= 2.03 J/g degree celcius, heat of evaporation of H2O (I) is 2.260 kJ/g)

Chemistry Physical Chemistry Level: Misc Level
Calculate the percentage by mass of nitrogen in ammonium perchlorate
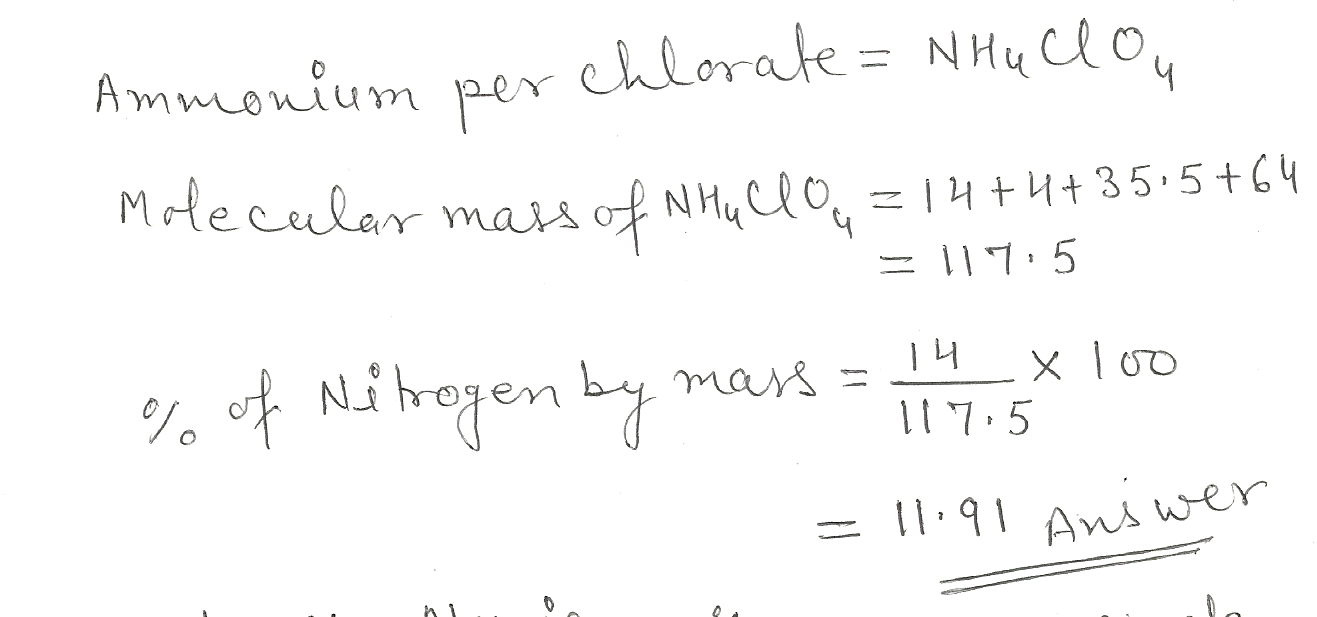
Chemistry Physical Chemistry Level: Misc Level
Heat capacity data can be used to estimate the reaction enthalpy at one temperature from its value at another. Predict the standard reaction enthalpy of 2 NO2 (g)>>>>NO4(g)at 100 degrees C from its value at 25 degrees C which is -112.2 kJ /mol

Chemistry Physical Chemistry Level: Misc Level
Camping gas is typically propane.The standard enthalpy of combustion of propane gas is -2220 kJ /mol and the standard enthalpy of vaporization of the liquid is + 15 kJ /mol.Calculate the standard enthalpy and the standard internal energy of combustion of the liquid.

Chemistry Physical Chemistry Level: Misc Level
Given that the enthalpy of combustion of graphite is -393.5 kJ/mol and that of diamond is-395.41 kJ /mol calculate the standard enthalpy of the C (s,graphite) >>>>C(s, diamond) transition.

Chemistry Physical Chemistry Level: Misc Level
The standard enthalpy of combustion of naphthalene is -5157 kJ /mol. Calculate its standard enthalpy of formation.

Chemistry Physical Chemistry Level: Misc Level
The enthalpy changes accompanying the dissociation of succesive bonds in N H 3(g) are 460, 390, and 314 kJ /mol respectively. a) What is the mean enthalpy of an N-H bond?
b) Do you expect the mean bond internal energy to be larger or smaller then the mean bond enthalpy?
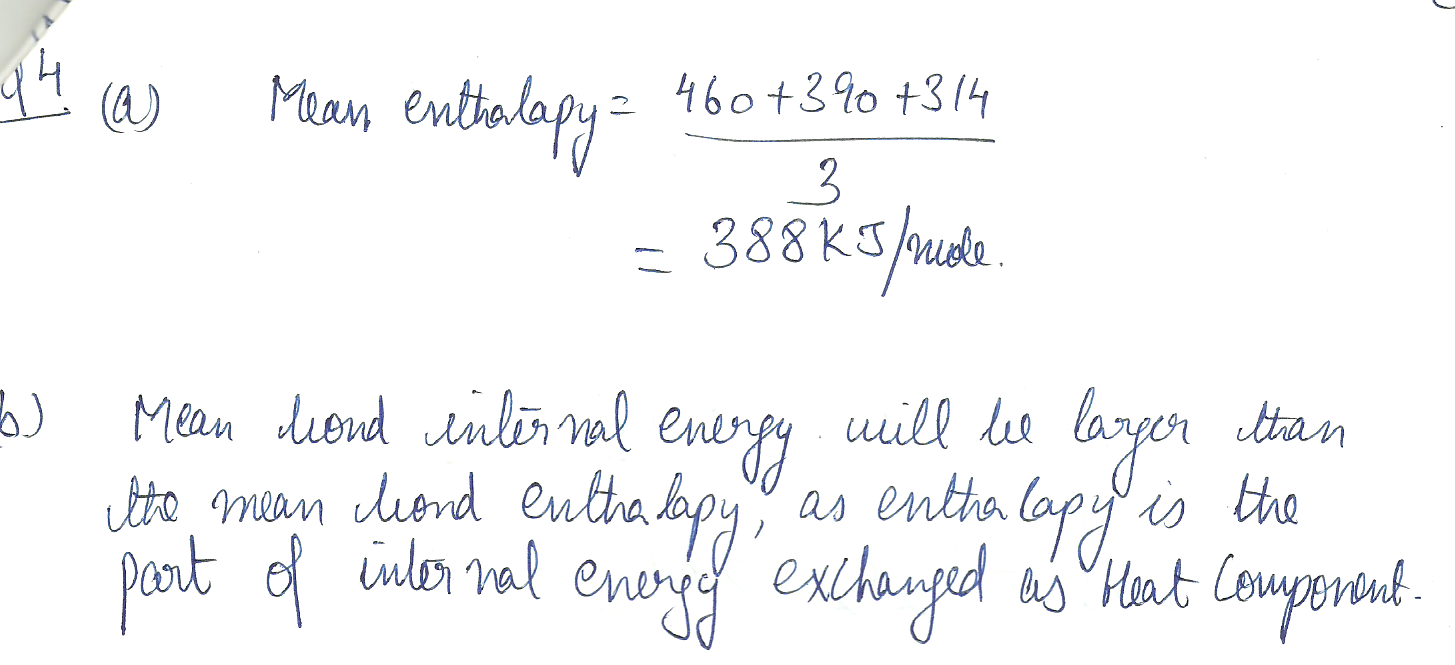
Chemistry Physical Chemistry Level: Misc Level
The vapour pressure of a sample of benzene is 53.0 k P a at 60.6 degrees C, but it fell to 51. 2 k P a when 0. 125 g of an organic compound was dissolved in 5.00 g of the solvent. Calculate the molar mass of the compound.

