Chemistry Inorganic Chemistry Level: Misc Level
Write the net ionic equation of the reaction between aqueous ammonia,NH4OH and sulfuric acid.

Chemistry Inorganic Chemistry Level: Misc Level
A 0.785 M solution of H2SO4 has been prepared as you described in question(1).Thereafter,50 mL of this solution was transferred to another container and diluted to 150 mL to prepare for titration.During titration, it was observed that it took 16.82 mL of the dilute sulfuric acid solution to titrate 25 mL of Na NaOH.What is the molarity of sodium hydroxide?

Chemistry Inorganic Chemistry Level: Misc Level
You are asked to preoare 500 mL of a 0.785 M solution of H2SO4. Concentrated sulfuric acid has a density of 1.8305 g/mL.
a) How many mL of H2SO4 do you need? b.) Describe how you will prepare the solution.

Chemistry Inorganic Chemistry Level: Misc Level
How many grams of the excess reagent are unreacted

Chemistry Inorganic Chemistry Level: Misc Level
Identify the limiting reactant
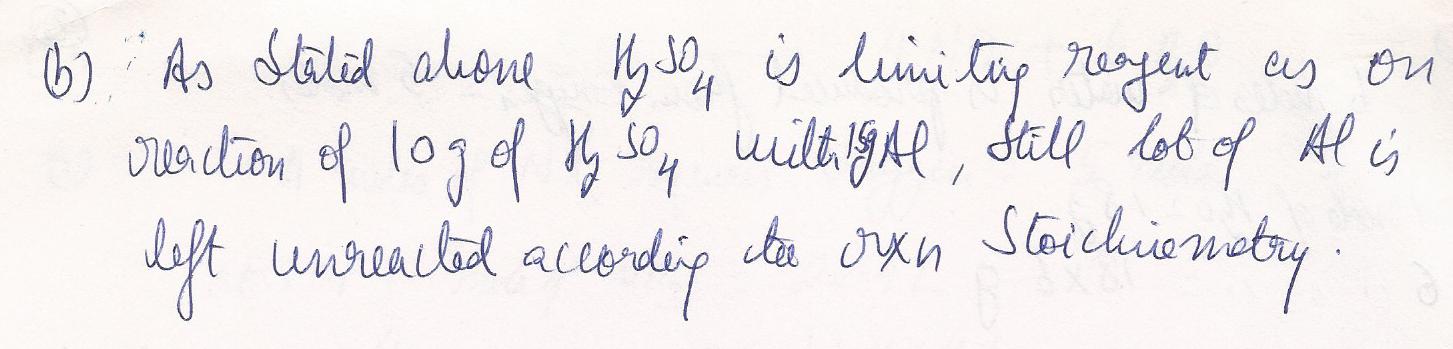
Chemistry Inorganic Chemistry Level: Misc Level
How many grams of aluminum sulfate are produced when reacting 15.00 g aluminum with 10.00 g of sulfuric acid
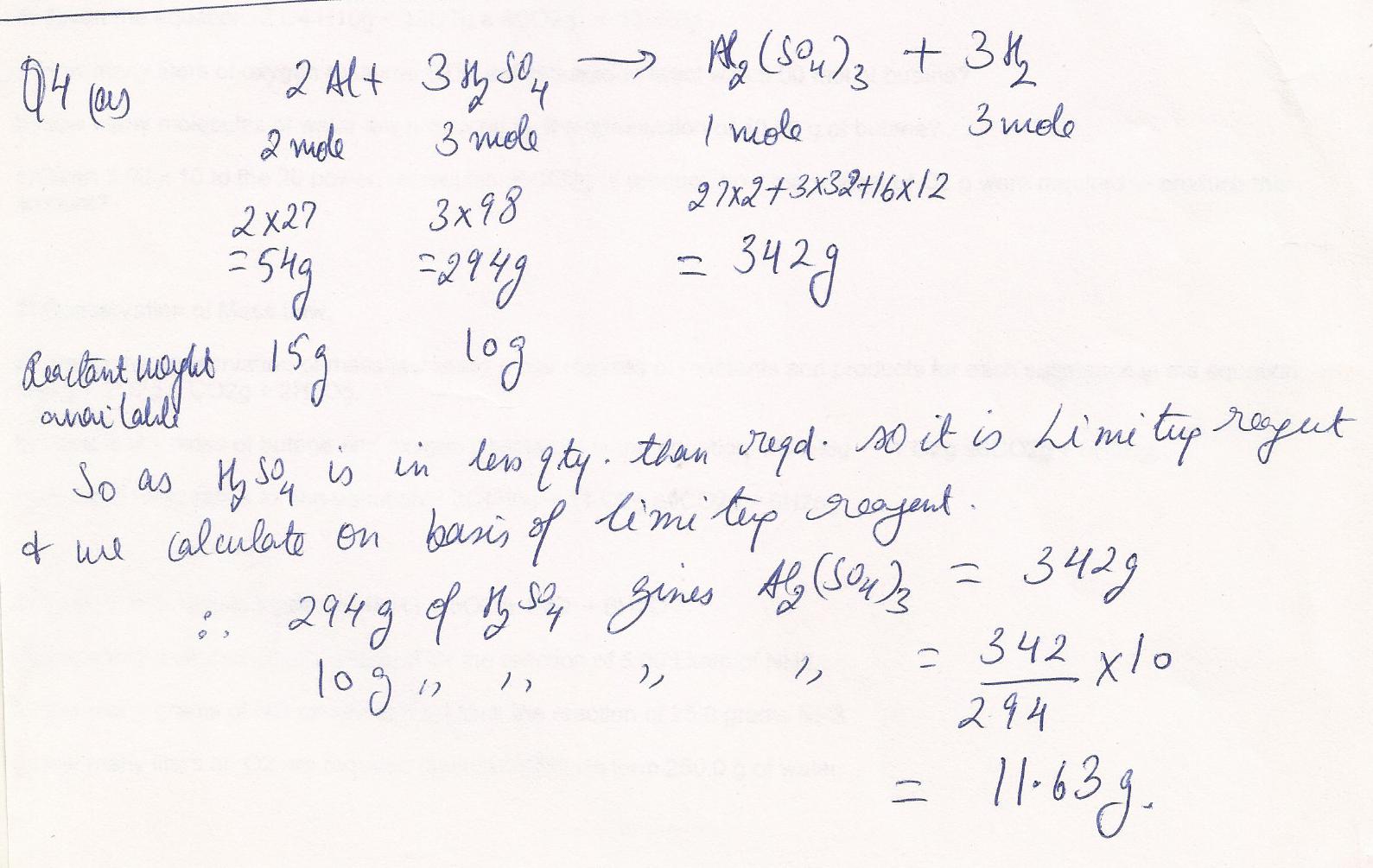
Chemistry Inorganic Chemistry Level: Misc Level
Given the equation ;2AI+3H 2SO4 ---> AI2(SO4)3+ 3H2

Chemistry Inorganic Chemistry Level: Misc Level
How many liters of O2 are required (assume STP) to form 250.0 g of water
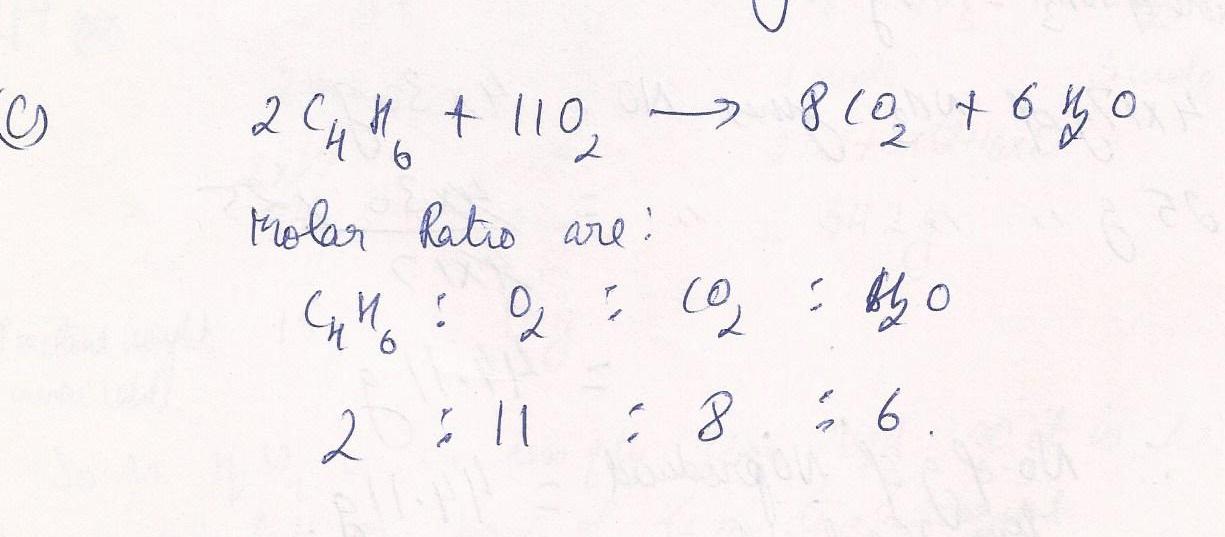
Chemistry Inorganic Chemistry Level: Misc Level
How many grams of NO can be formed from the reaction of 25.0 grams NH3

Chemistry Inorganic Chemistry Level: Misc Level
How many moles of O2 are required for the reaction of 5.00 Liters of NH3.
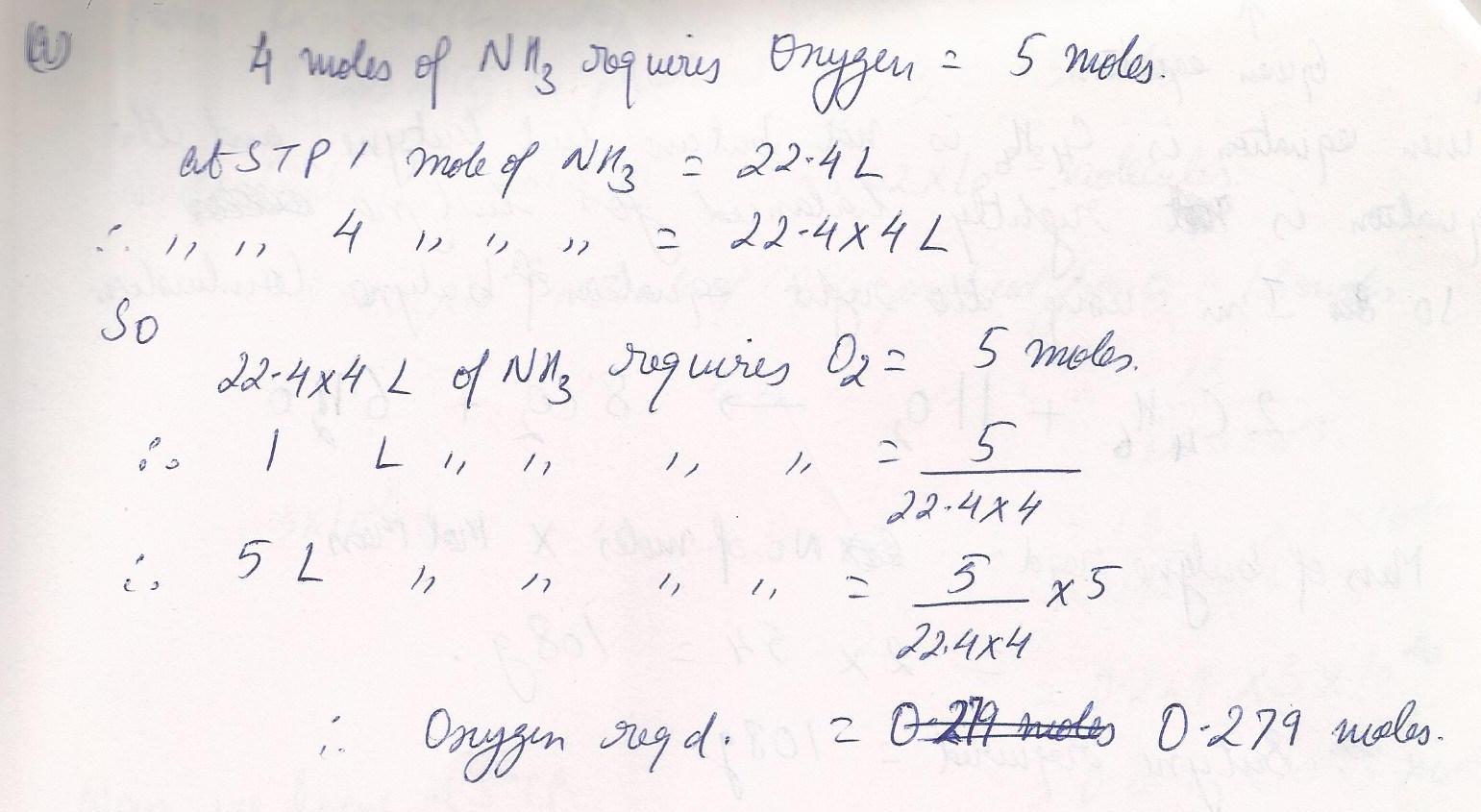
Chemistry Inorganic Chemistry Level: Misc Level
Write all mole ratios for the equation; 2C4H6+11O2 ----> 8CO2+ 6H2 O
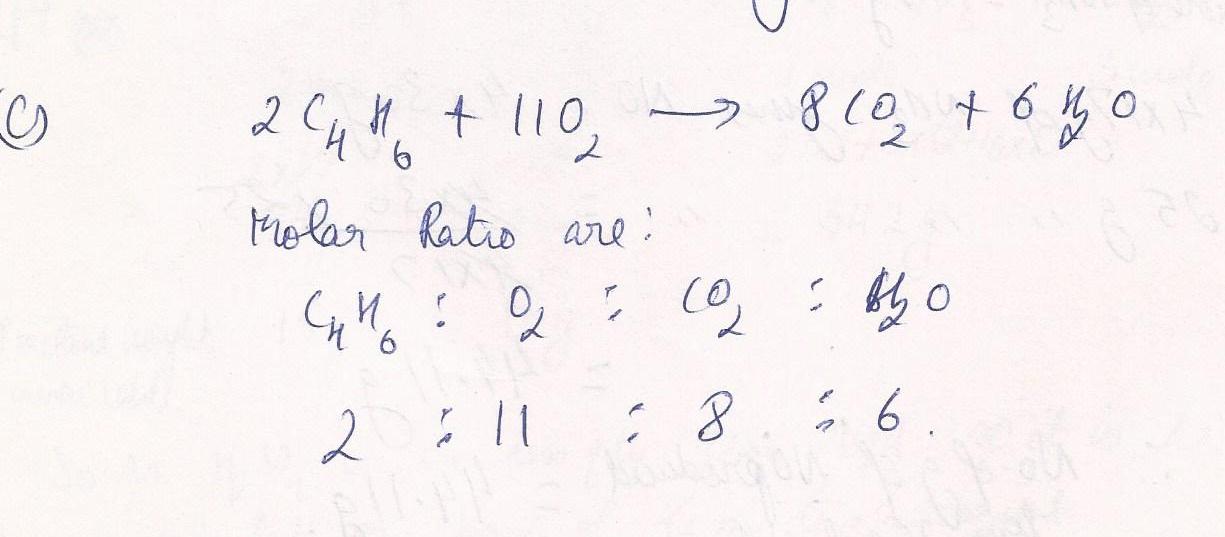
Chemistry Inorganic Chemistry Level: Misc Level
What is the mass of butane and oxygen (reactants) in the equation; 2C4H6+11O2 ----> 8CO2+ 6H2 O

Chemistry Inorganic Chemistry Level: Misc Level
Verify the conservation of mass law using molar masses of reactants and products for each substance in the equation;
CH4+2O2 ---->CO2 + 2H2O

Chemistry Inorganic Chemistry Level: Misc Level
Given 3.00 x1020 molecules of CO2 are produced, how many liters of O2 were required to produce this amount?

Chemistry Inorganic Chemistry Level: Misc Level
How many molecules of water are produced by the combustion of 40.00 g of butane?

