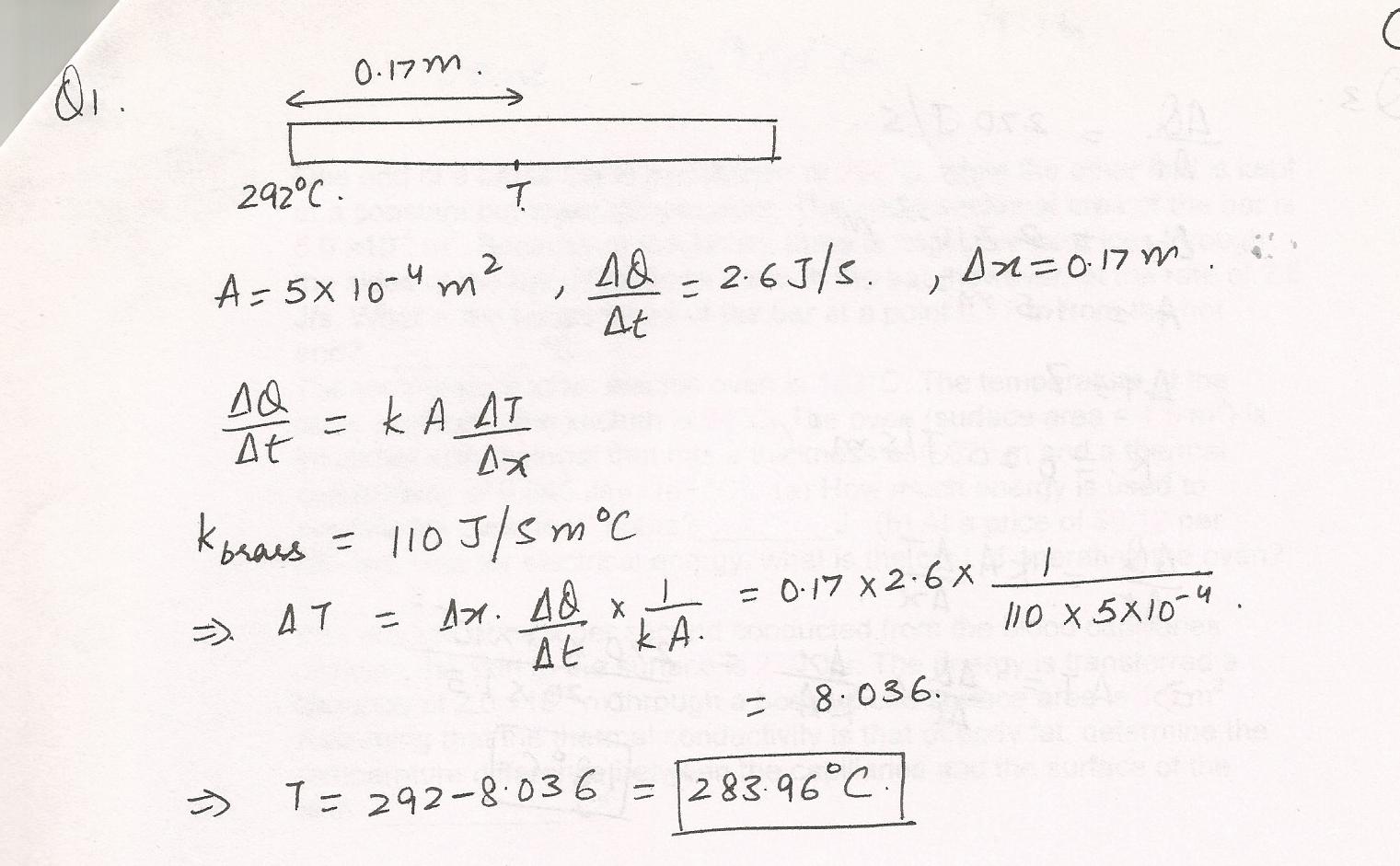Physics Heat & Thermodynamics Level: Misc Level
Calculate the amount of energy required to heat 21.7 g of water from 18.2 degrees Celcius to 39.7 degrees celcius.Then calculate the amount of energy required to heat 21.7 g of iron from 18.2 degrees Celcius to 39.7 degrees celcius. Does heating the iron take more or less energy than heating the same mass of water?
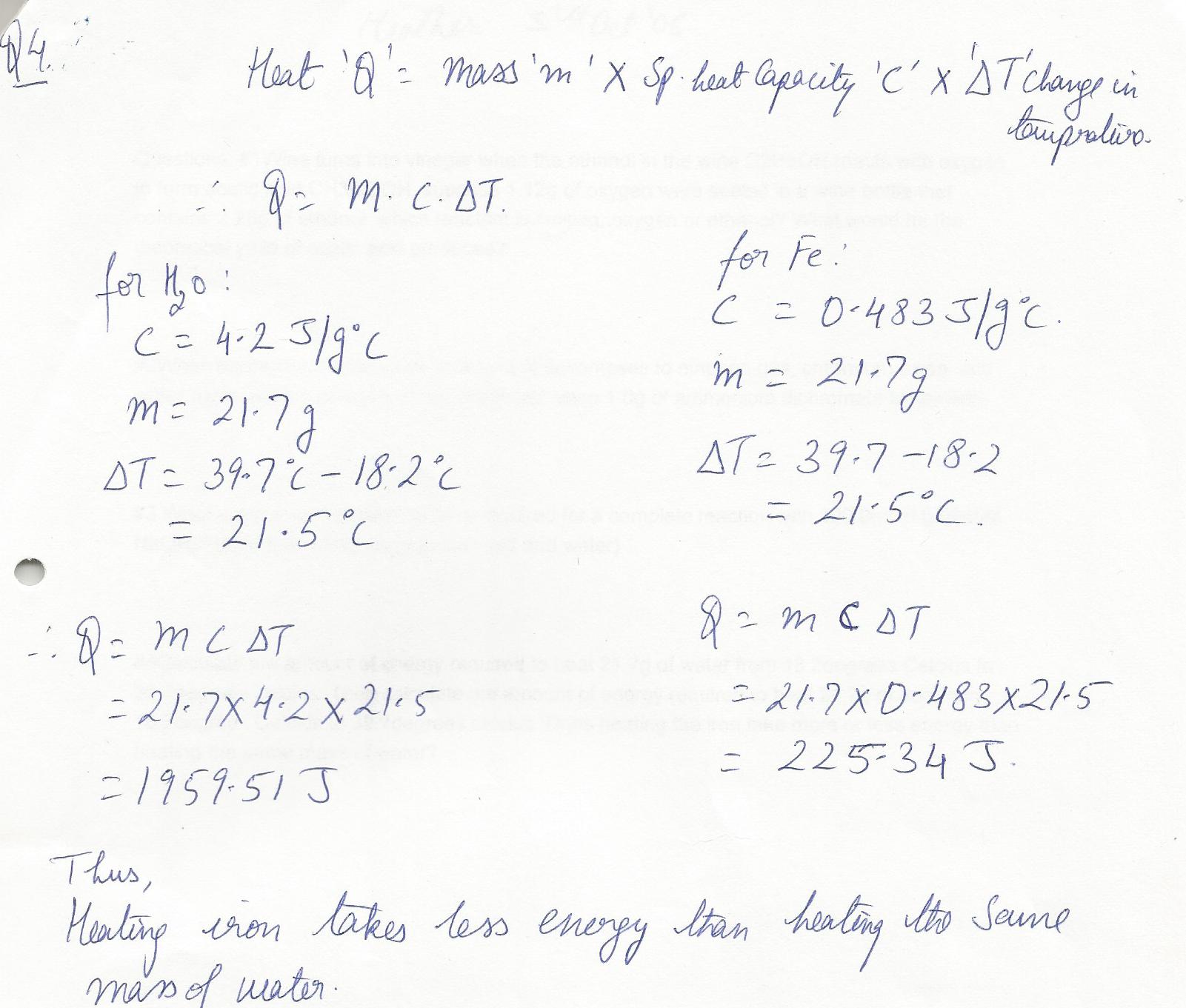
Physics Heat & Thermodynamics Level: Misc Level
A Carnot refrigeration is used to make 1 kg of ice at-10 C from 1 kg of water at 20 C, which is also the temperature of the kitchen. How many joules of work must be done?

Physics Heat & Thermodynamics Level: Misc Level
Three designs for a heat engine to operate between 450 K and 300 K are proposed.Design A is claimed to require a heat input of 2 kJ for each kJ of work output, design B a heat input of 3 kJ, and design C a heat input of 4 kJ. Which design would you choose and why?

Physics Heat & Thermodynamics Level: Misc Level
A gas expands by 1 L at a constant pressure of 2 bars. During the expansion 500 J of heat is added. Find the change in the internal energy of the gas.

Physics Heat & Thermodynamics Level: Misc Level
A cylinder containing monatomic gas and closed by a movable piston.The cylinder is submerged in an ice-water mixture.The piston is quickly pushed down from the top postion closer to the bottom. The piston is held there until the gas is again at 0 degrees celcius and then it is slowly raised back up to it,s orginal position. If 100 grams of ice are melted during the cycle, how much work has been done on the gas?
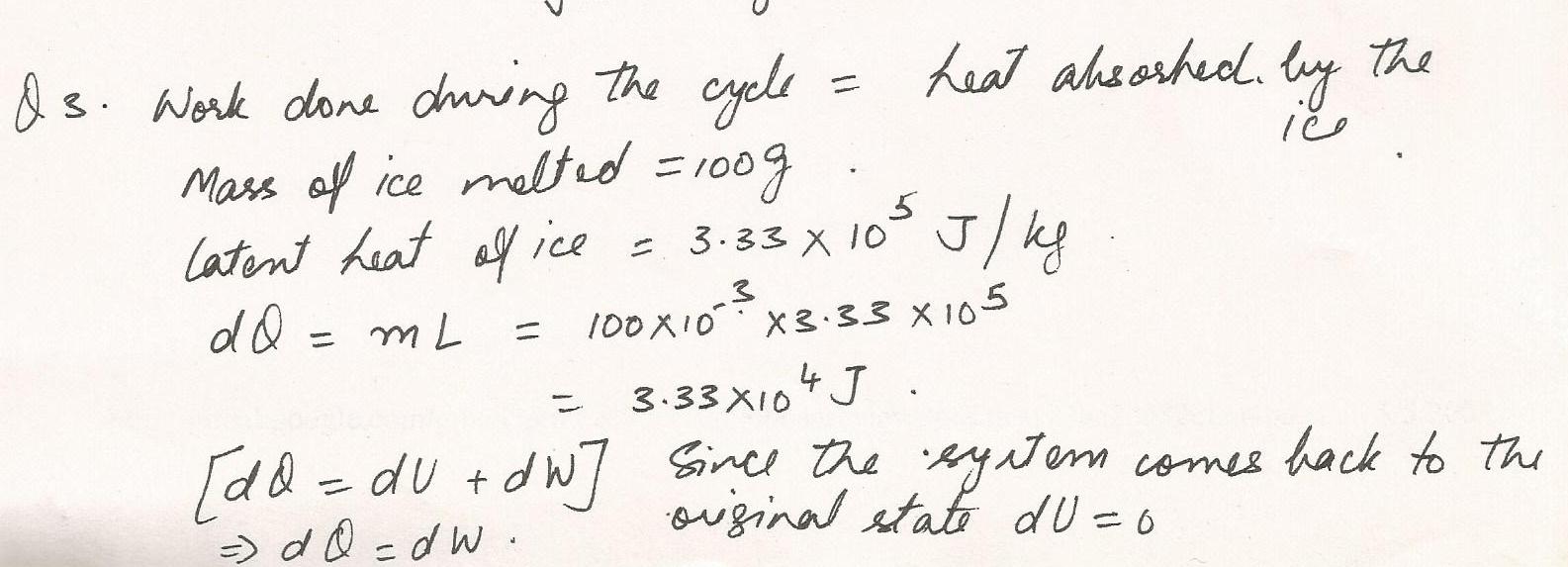
Physics Heat & Thermodynamics Level: Misc Level
Why must a room air conditioner be set partially out of a window? Why can,t it just be set in the center of a room and plugged in? Note that we are talking about an air conditioner, not a water evaporative cooler (swamp cooler).

Physics Heat & Thermodynamics Level: Misc Level
When an automobile engine overheats and the radiator water begins to boil, the car can still be driven some distance before catastrophic engine damage occurs. Why ? What determines the onset of really disastrous overheating?

Physics Heat & Thermodynamics Level: Misc Level
At 20.0 degrees Celius a copper disk is 10.00 cm in diameter and a hole in the disk is 0.4000 cm in diameter. what are the diameters of the disk and the hole at 200 degress Celius? The coefficient of the linear expansion of copper is 1.70x10^-5/(degrees Celius)

Physics Heat & Thermodynamics Level: Misc Level
A gas contains molecules made with two different atoms. Measurements of the specific heat give results as stated.From T (sub-1) to T (sub-2) Cv/R=11.5, from T (sub-2) to T (sub-3) Cv/R=2.5, from T (sub-3) to T (sub-(k) Cv/R=3.5 Describe the value of the three different results for Cv in the given temperature ranges as well as you are able to.

Physics Heat & Thermodynamics Level: Misc Level
A gas contains molecules made with two different atoms.Measurements of the specific heat give results as stated.From T (sub-1) to T (sub-2) Cv/R=31.5 from T (sub-2) to T (sub-3) Cv/R=2.5, from T (sub-3) to T (sub-(k) Cc /R=3.5 Describe the value of the three different results for Cv in the given temperature ranges as well as you areable to.

Physics Heat & Thermodynamics Level: Misc Level
A small meteorite comprised of iron with mass 1.32x10^3 kg enters Earths atmosphere from space and crashes into Lake Camplain (a body of fresh water, which has a total mass of 8.56x10^8 kg ).The entry into Earths atmosphere increases the temperatureof the meteorite to 3.24 x10^3 degrees celius just before it comes apart in small pieces and impacts he water at a speed of 3500 m/s above the deepest part of the lake.The temperature of the lake is a chilly 10 degrees celius just before the impact. what is the final temperature of the system of the lake and meteorite? For this purpose asume the lake bottom is a good thermal insulator.

Physics Heat & Thermodynamics Level: Misc Level
The air temperature over a lake is -10 degress Celius, while the water below the surface of the ice(now 1..00 cm thick) is at 4 degrees celius. What is the speed at which the ice layer on the lake thickens (measured in m/s or some multiple thereof ) ? Information you may need; The thermal conductivity of ice is 1.6 w/(mk), the specific heat of ice is 2000 J/(kg degrees Celius),that of water is 4186 J/(kg degrees celius) and the latent heat of fusion of water is 334 kJ /kg.

Physics Heat & Thermodynamics Level: Misc Level
The amount of heat per second conducted from the blood capillaries beneath the skin to the surface is 270 J/s. The energy is transferred a distance of 2.0x10-3 m through a body whose surface area is 1.5 m2.Assuming that the thermal conductivity is that of body fat, determine the temperature difference between the capillaries and the surface of the s %C
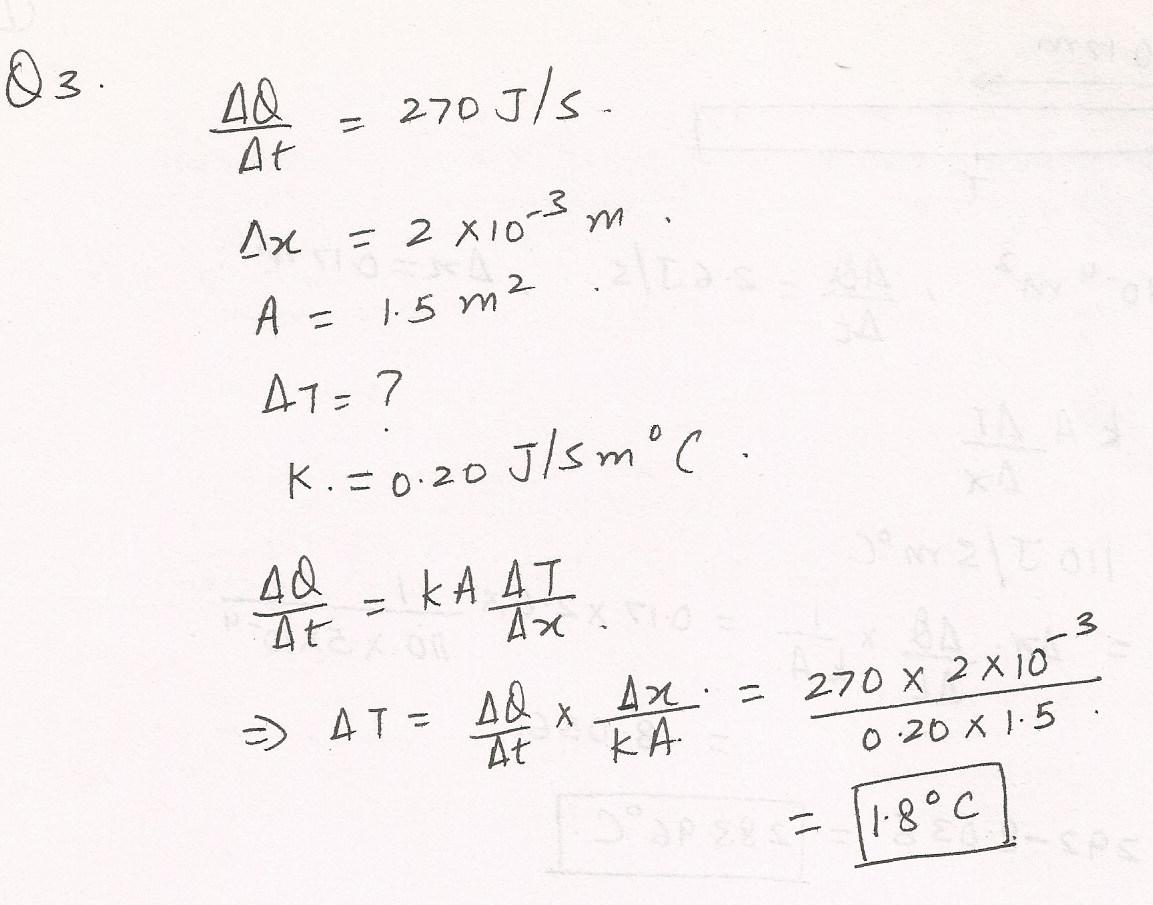
Physics Heat & Thermodynamics Level: Misc Level
The temperature in an electric oven is 163%C. The temperature at the outer surface in the kitchen is 54%C. The oven (surface area=1.5 m2) is insulated with material that has a thickness of 0.022 m and a thermal conductivity of 0.045 J/(s.m.%C).(a) How much energy is used to operate the oven for 7 hours?--J (b) At a price of $0.12 per kilowatt hour for electrical energy, what is the cost of operating the oven$--
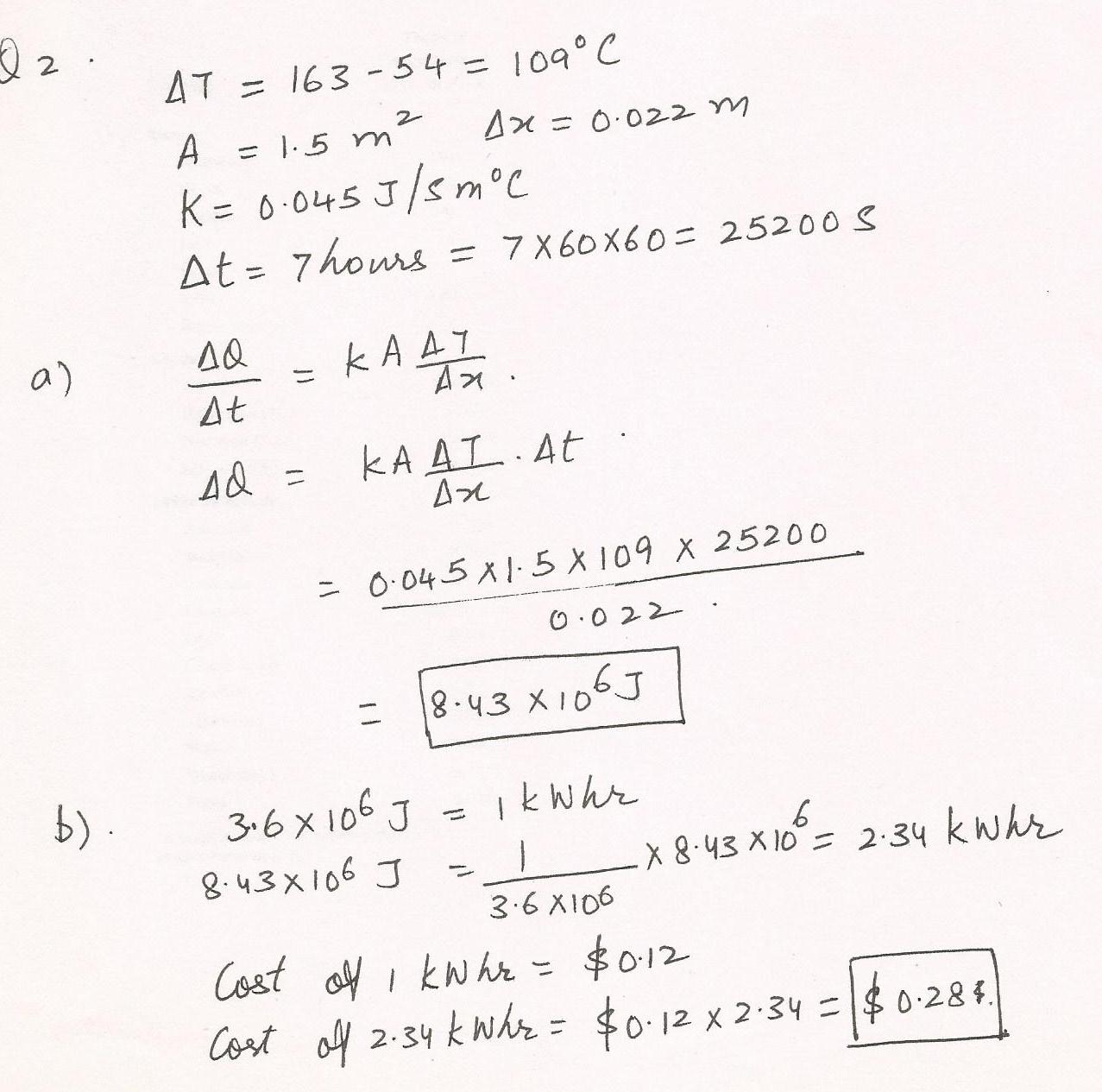
Physics Heat & Thermodynamics Level: Misc Level
One end of a brass bar is maintained at 292%C, while the other end is kept at a constant but lower temperature. The cross-sectional area of the bar is 5.0 x 10-4 m2. Because of insulation, there is negligible heat loss through the sides of the bar. Heat flows through the bar, however, at the rate of 2.6 J/s. What is the temperature of the bar at a point 0.17 m from the hot end?---%C