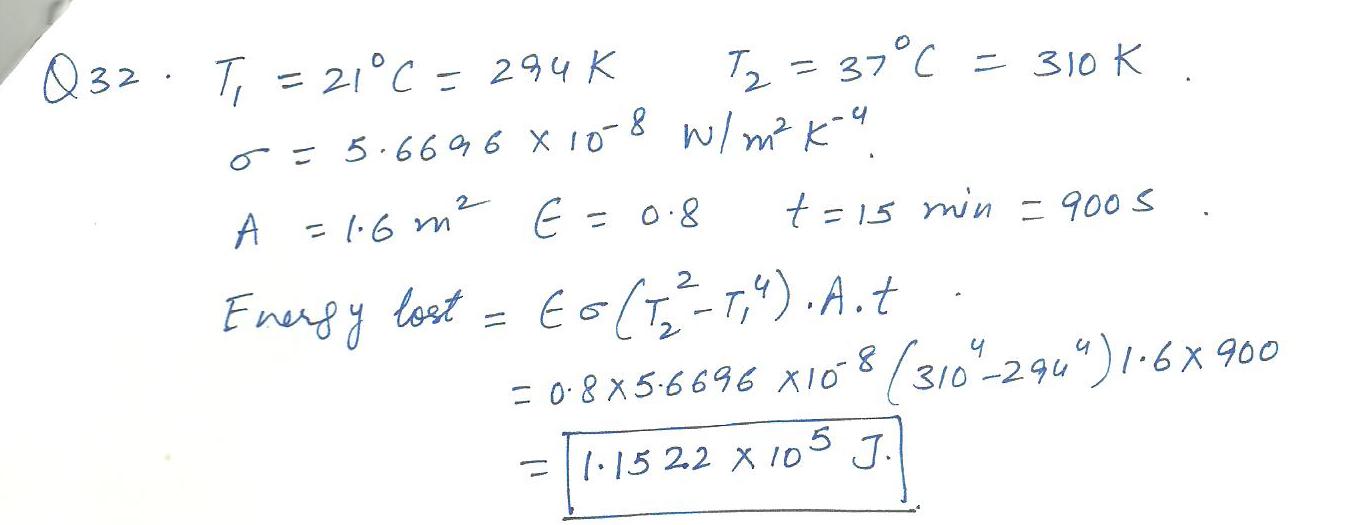Physics Heat & Thermodynamics Level: Misc Level
0.16 kg of water at 85 celcius is pured into an insulated cup containing 0.206 kg of ice initally at 0 degrees. calculate the mass of liquid when the system reaches thermal equallibrium. (in kg)
a)2.93 all to power of-1 b) 3.31 c) 3.74 d)4.23 e)4.78 f)5.40 g)6.10 h)6.89
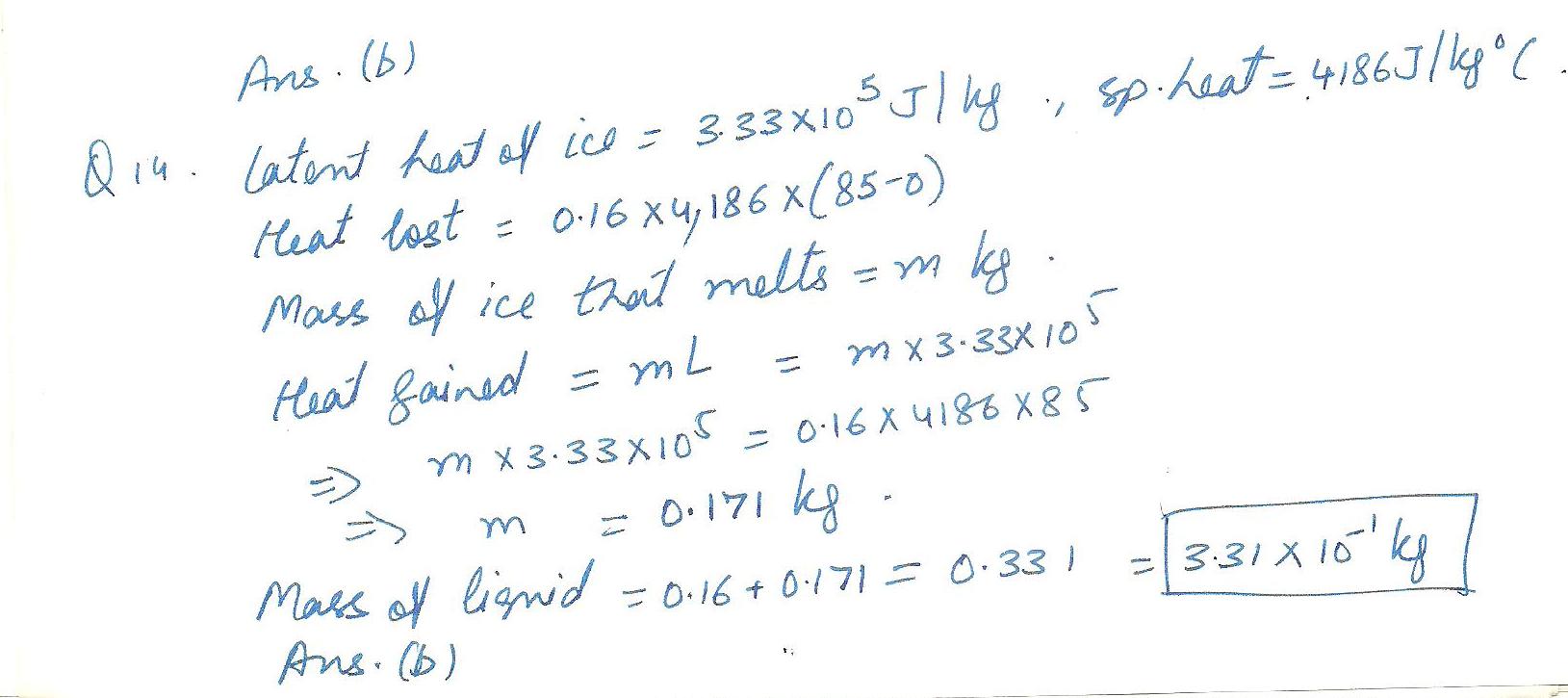
Physics Heat & Thermodynamics Level: Misc Level
a hot 800 kelvin and a cold 200 kelvin object are connected by two aluminum bars as shown in the attaached file. considering the left configuration only, lowering the temperature of the 800 kelvin block to 400 kelvin will reduce the rate of heat transfer of --
a)1/4 b)1/2 c)1/3
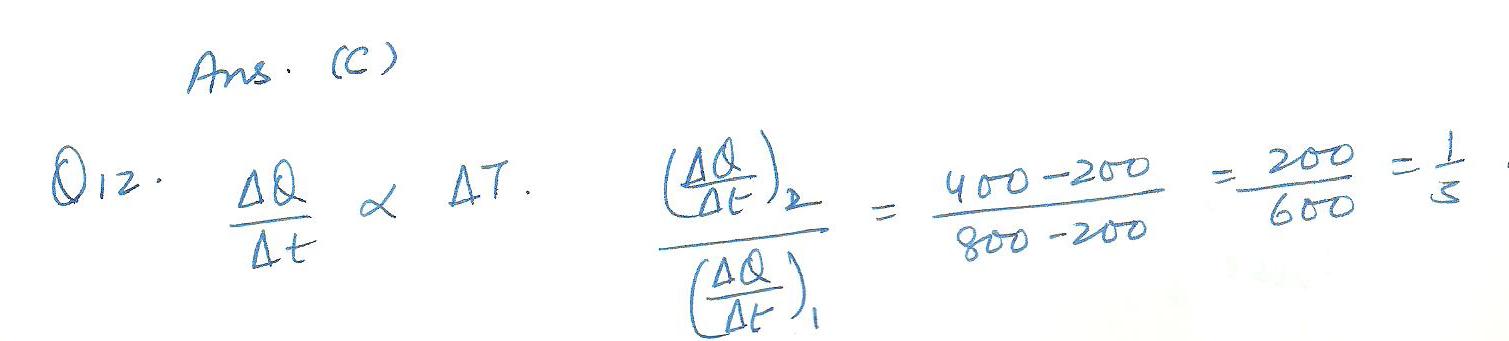
Physics Heat & Thermodynamics Level: Misc Level
A 200-g piece of platinum is placed inside a furnace until it is in thermal equirium. The platinum is then placed in a 100-g aluminum calormeter contaning 400 g of water at 5.00 c.If the final temperature of the water is 10.0 find the temperature of the fumance.

Physics Heat & Thermodynamics Level: Misc Level
A 100-g aluminum calorimeter contains 200g of water at 15.0 c. if 100g of lead at 50.0c and 60.0g of copper at 60.0c are placed un the calorimeter, what is the final temp in the calorimeter?

Physics Heat & Thermodynamics Level: Misc Level
In a particular biological reaction taking place in the body at 37 degrees C, the change in enthalpy was- 125 kJ/mol and the change in entropy was-126 J/K mol (a) Calculate the change in Gibbs energy. (b) is the reaction spontaneous?(c)Calculate the total change in entropy of the system and surroudings.

Physics Heat & Thermodynamics Level: Misc Level
The enthalpy of vaporization of chloroform CHCI3, is 29.4 kJ/mol at its normal boiling point of 334.88 K (a) calculate the entropy of vaporization of chloroform at this temperature. (b) What is the entropy change in the surroundings?

Physics Heat & Thermodynamics Level: Misc Level
The enthalpy of graphite >>>> diamond phase transition, which under 100 kbar occurs at 2000 K, is +1.9 kJ/mol. Calculate the entropy of transition.

Physics Heat & Thermodynamics Level: Misc Level
What is the change in entropy 100g of water when it is heated from room temperature (20 degreesC) to body temperature (37 degress C) Use Cp, m=75.5 J /K mol
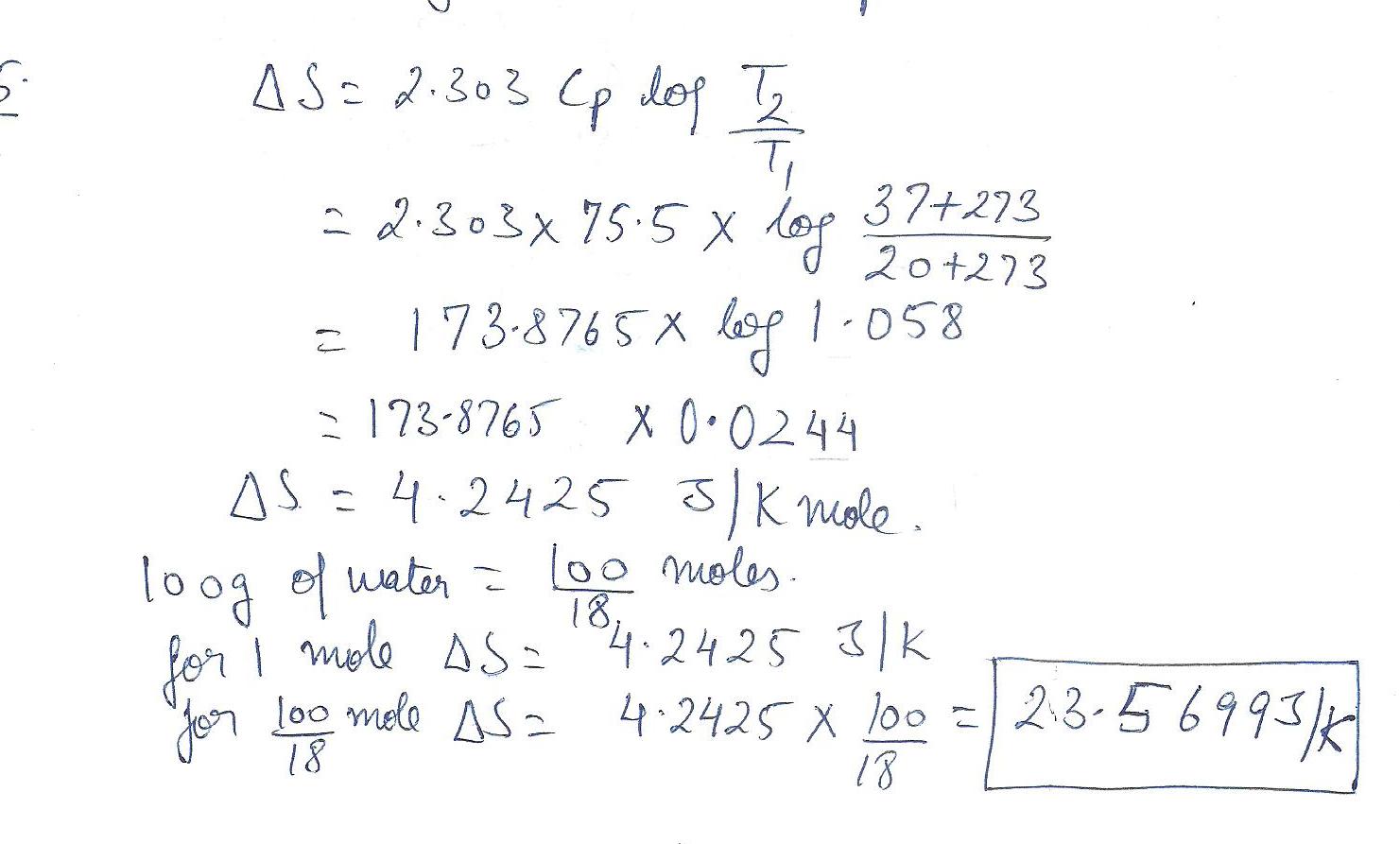
Physics Heat & Thermodynamics Level: Misc Level
Whenever a gas expands -when we exhale, when a flask is opened, and so on -the gas undergoes an increase in entropy. A sample of methane gas of mass 25 g at 250 K and 185 kPa expands isothermally and (a)reversble, (b) ireversible until its pressure is 2.5 kPa. Calculate the change in entropy of the gas.

Physics Heat & Thermodynamics Level: Misc Level
A sample of carbon diooxide that initially occupies 15.0 dm cubed at 250 K and 1.00atm is compressed isothermally. into what volumemust the gas be compressed to reduce entropy by 10.0 J/K?

Physics Heat & Thermodynamics Level: Misc Level
Calculate the change in molar entropy when carbon dioxide expands isothermally from 1.5 dm cubed to 4.5 dm cubed.
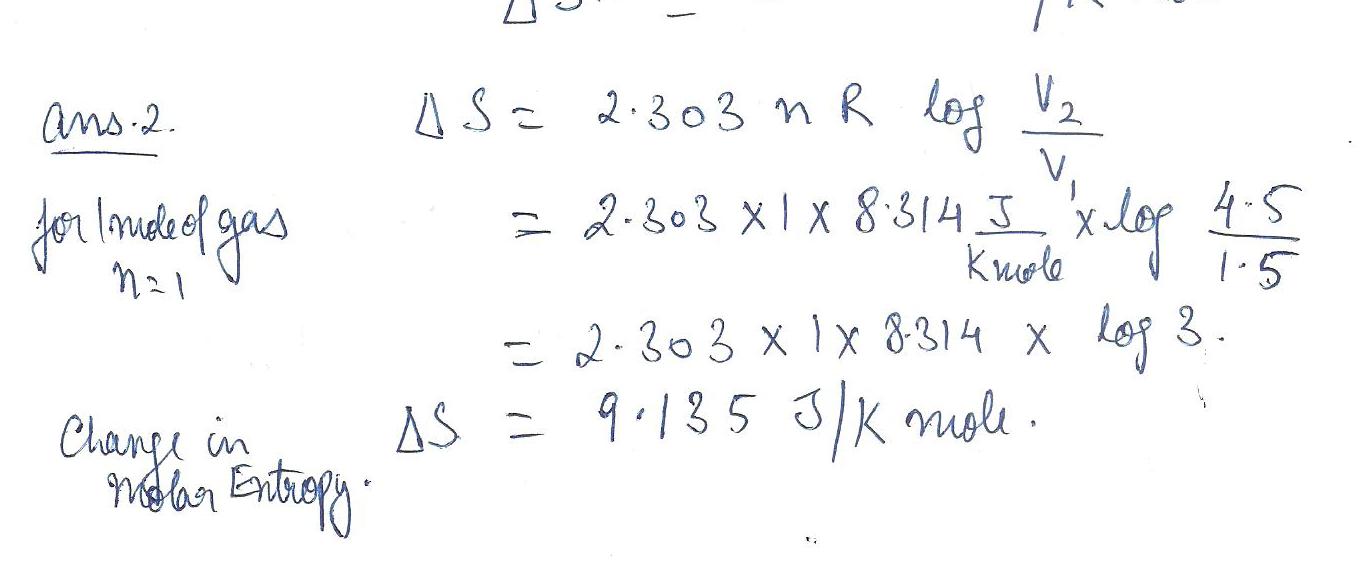
Physics Heat & Thermodynamics Level: Misc Level
Consider a solid steel plate with a hole through its center. When the plates temperature is decreased the diameter of the hall will:

Physics Heat & Thermodynamics Level: Misc Level
How much heat is added 500g of water to raise its temperature from 15 degrees c to 85 degrees c?
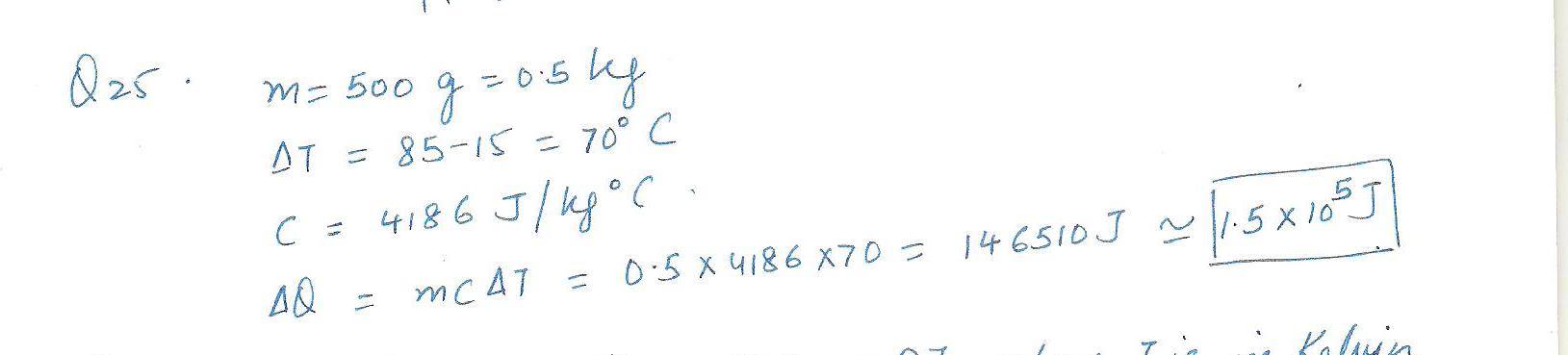
Physics Heat & Thermodynamics Level: Misc Level
Aluminum has a positive co-efficient of thermal expansion consider a round hole that has been drilled in a large sheet of Aluminum. As the temperature increases and the surrounding metal expands, the hole diameter will:
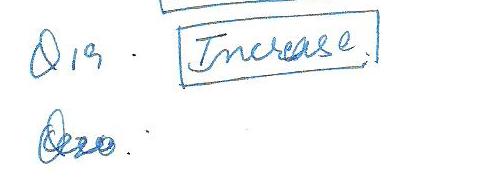
Physics Heat & Thermodynamics Level: Misc Level
Given:o =5.6696 x10^-8 W/m^2x K^4: An unclothed student is in 21 * C room If the skin temperature of the student is 37*C ,how much heat is lost from his body in 15 min, assuming that emissivity of the skin is 0.8 and the surface area of the student is 1.6 m^2. Answer in units of J.