Physics Heat & Thermodynamics Level: Misc Level
If 0.10 kg of ice at 0% C is added to 0.60 kg of water at 20 % C What is the final temperature of the water?
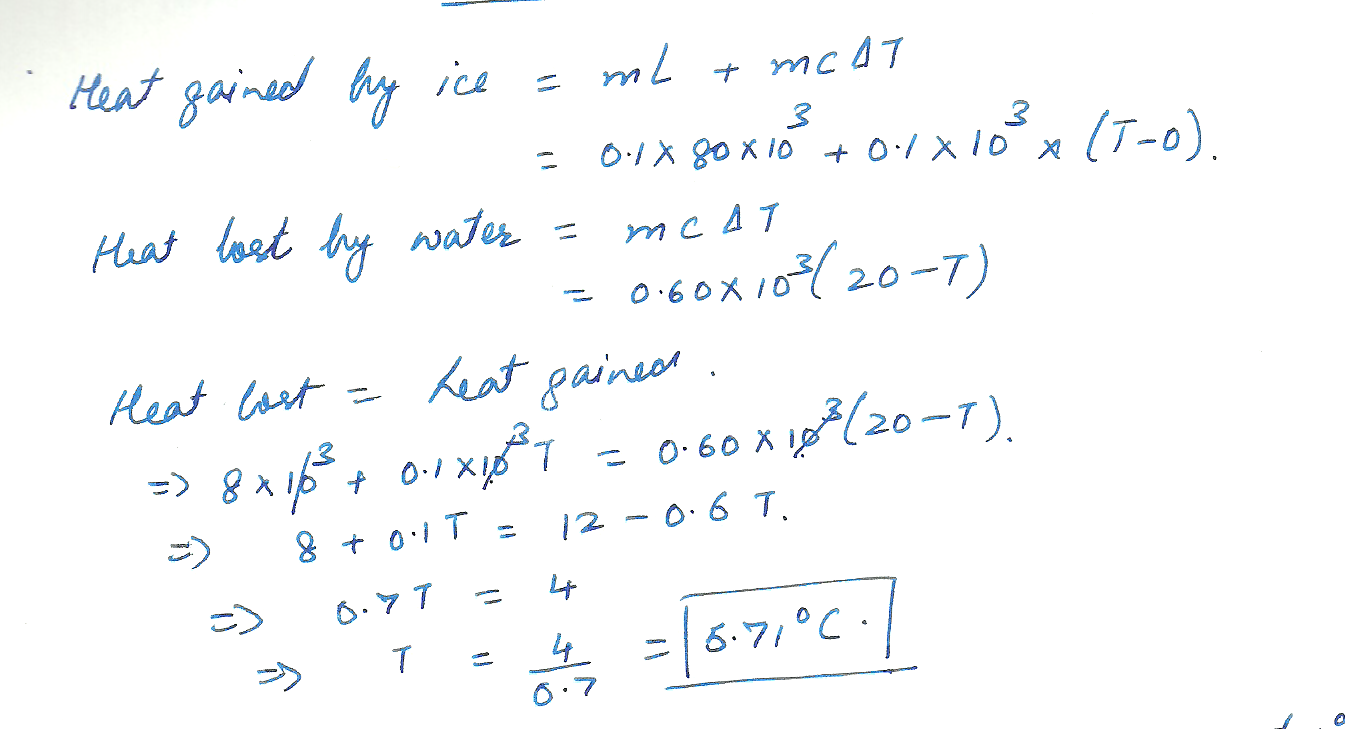
Physics Heat & Thermodynamics Level: Misc Level
Two grams of liquid water are at 0 o C, and another five grams are at 100 oC.Heat is removed from the water at 0 oC, completely freezing it at 0 oC.This heat is then used to vaporize some of the water at 100 oC. What is the mass (in grams) of the liquid water that remains?

Physics Heat & Thermodynamics Level: Misc Level
A woman finds the front windshield of her car covered with ice at 12.4 oC .The ice has a thickness of 4.35 x 10-4 m,3.and the windshield has an area of 1.25 m2. The density of ice is 917 kg /m3.How much heat is required to melt the ice?

Physics Heat & Thermodynamics Level: Misc Level
The latent heat of vaporizaiton of H2O at body temperature (37.0 oC ) is 2.42 x106 J/kg. To cool the body of a 72 kg jogger (average specific heat capacity =3500 J/(kg oC ) by 1.2 C %, how many kilograms of water in the from of sweat have to be evaporated?
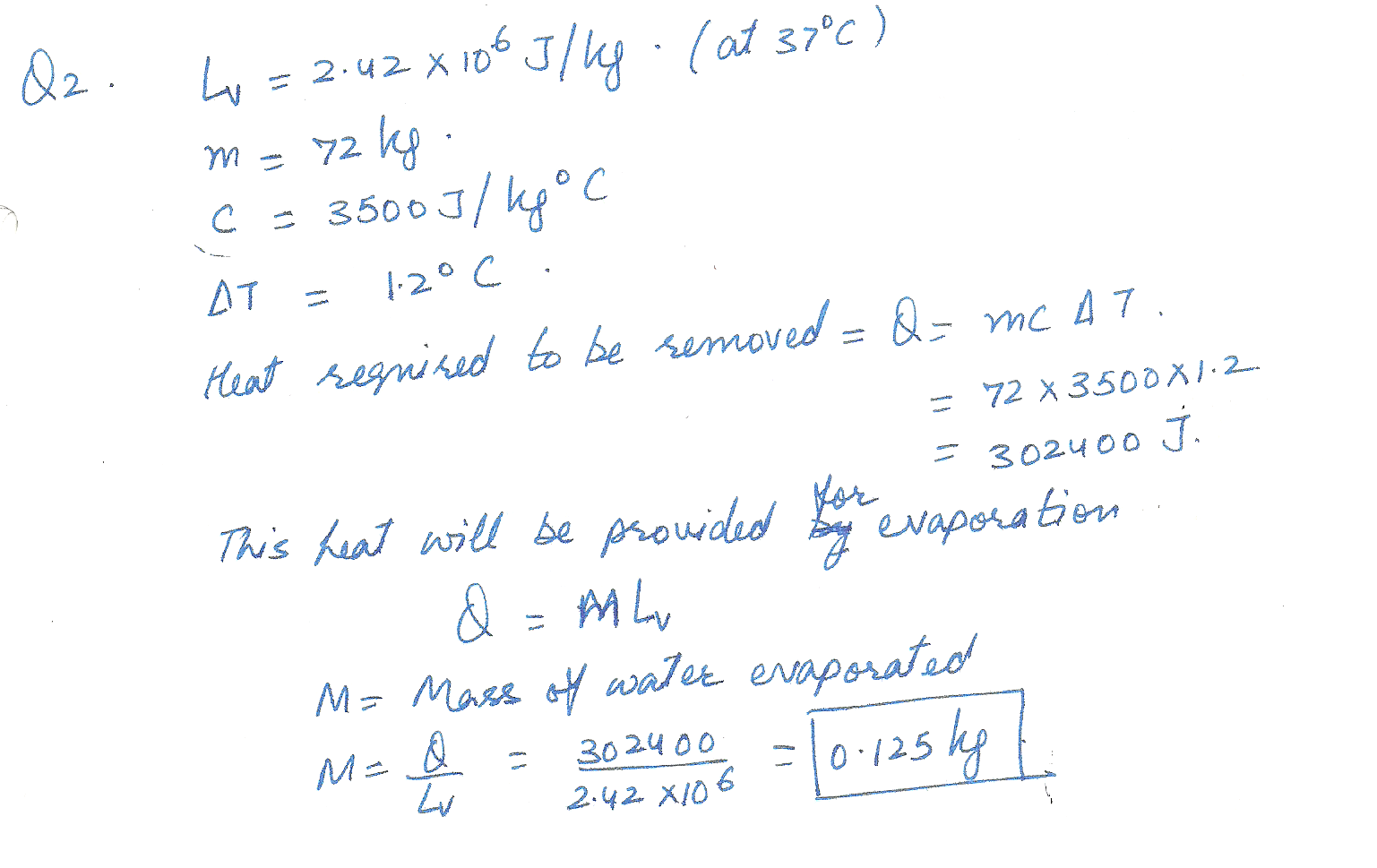
Physics Heat & Thermodynamics Level: Misc Level
At a fabrication plant, a hot metal forging has a mass of 79 kg and a specific heat capacity of 430 J/ (kg.% C).To harden it, the forging is immersed in 710 kg of oil that has a temperature of 32 % C and a specific heat capacity of 3000 J/ (kg.% C). The final temperature of the oil and forging at thermal equilibrium is 46 %C. Assuming that heat flows only between the forging and the oil, determine the initial temperature of the forging.

Physics Heat & Thermodynamics Level: Misc Level
An immersion heater has a power rating of 900 watts. it is used to heat water for coffee. how many minutes are required to heat 11.98 liters of water from room temp (20 degrees celcius) to 90 celcius?
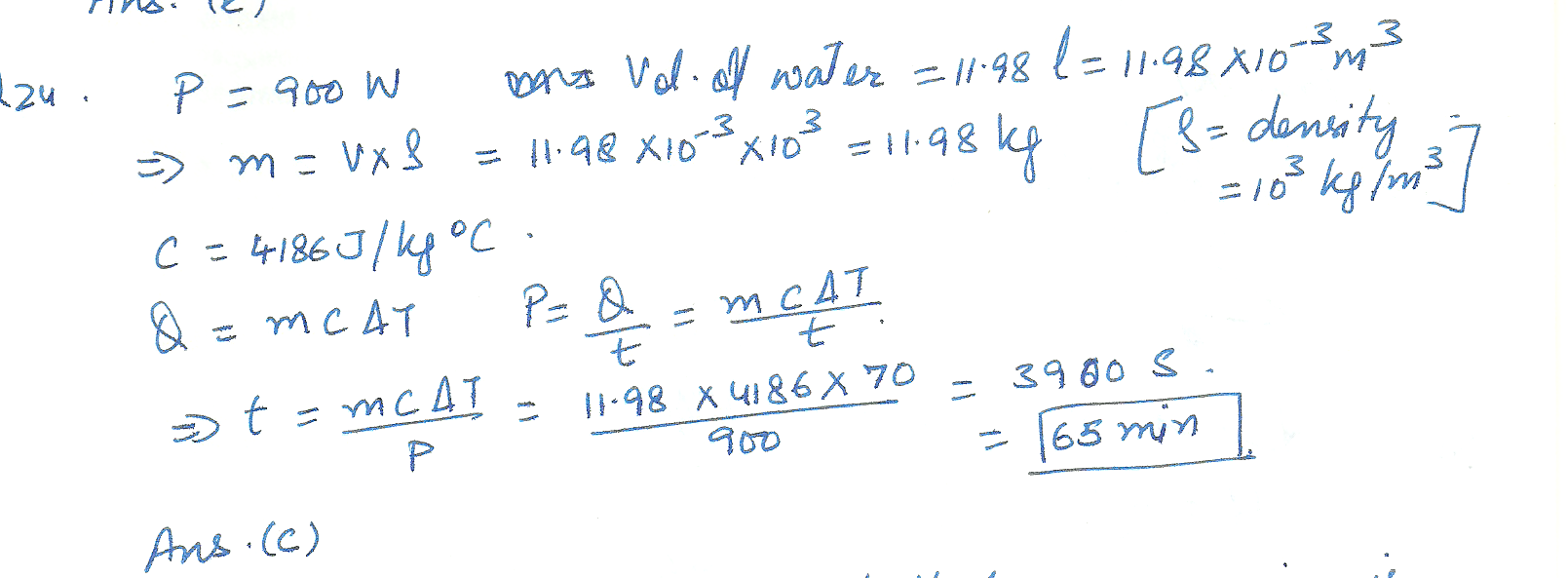
Physics Heat & Thermodynamics Level: Misc Level
How much would you have to raise the temperature of a copper wire (originally at 20.0 %C) to increase its resistance by 5.00 percent?
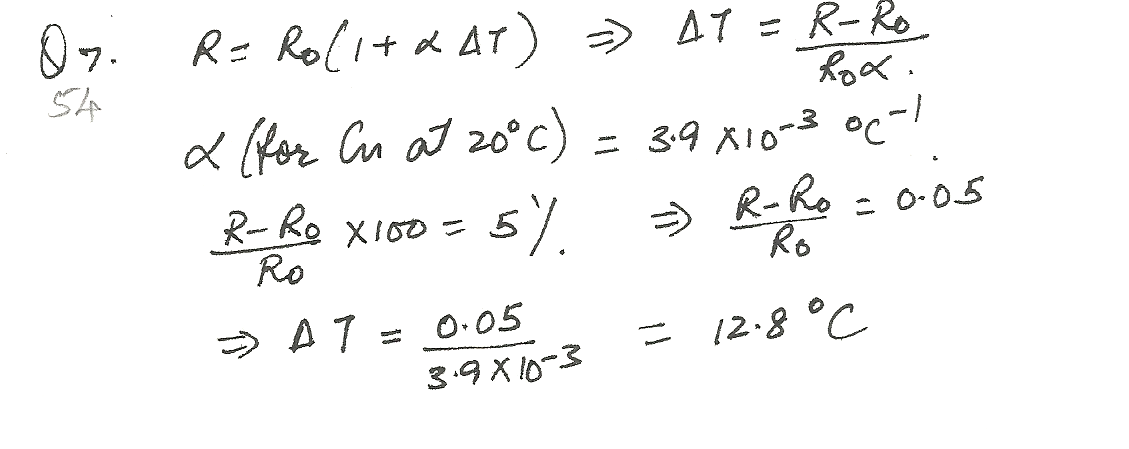
Physics Heat & Thermodynamics Level: Misc Level
2 objects are added to a container holding 200 g of 20 deg C ethanol. The hot object has a mass of 100 g and is removed from a pot of 212 deg F water, its specific heat being 1 /5 that of water.The other object is taken from a 263 K freezer, Its mass is 75 g and its specific heat heat 30 % that of water. What will be the final equilibrium temperature of the mixture?
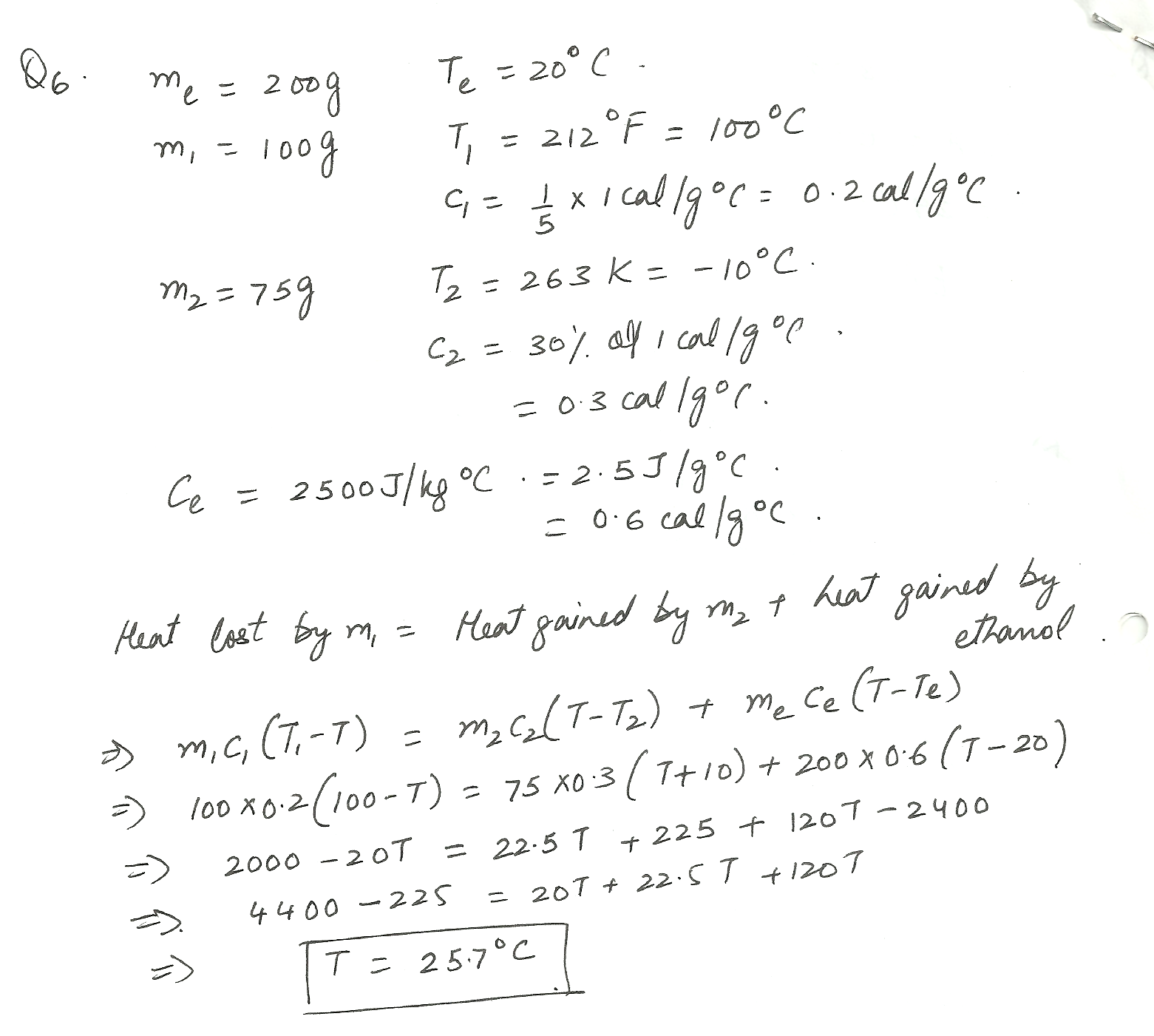
Physics Heat & Thermodynamics Level: Misc Level
Surface area of a city is 45 square miles with a snow density of 15% that of water . Average depth of snow is 13 inches. What is the volume in cubic meters of snow covering the city?How much energy in both joules and kcal would be needed to turn this snow (32 deg F) into water? Assume 9 hours of sunlight and average sunlight intensity is 400 W/meter squared. Also assume that 50% of this energy is absorbed by the snow. How many hours of sunlight is needed to melt the snow?

Physics Heat & Thermodynamics Level: Misc Level
Well insulated wall temperature of room A is 20 deg Celsius, and poorly insulated wall temperature of room B is 12 deg Celsius. Body surface temperature is 29 deg Celsius with a surface area of 1.8 meters squared and emissivity of 0.9 what is the man's radiant loss in each house?
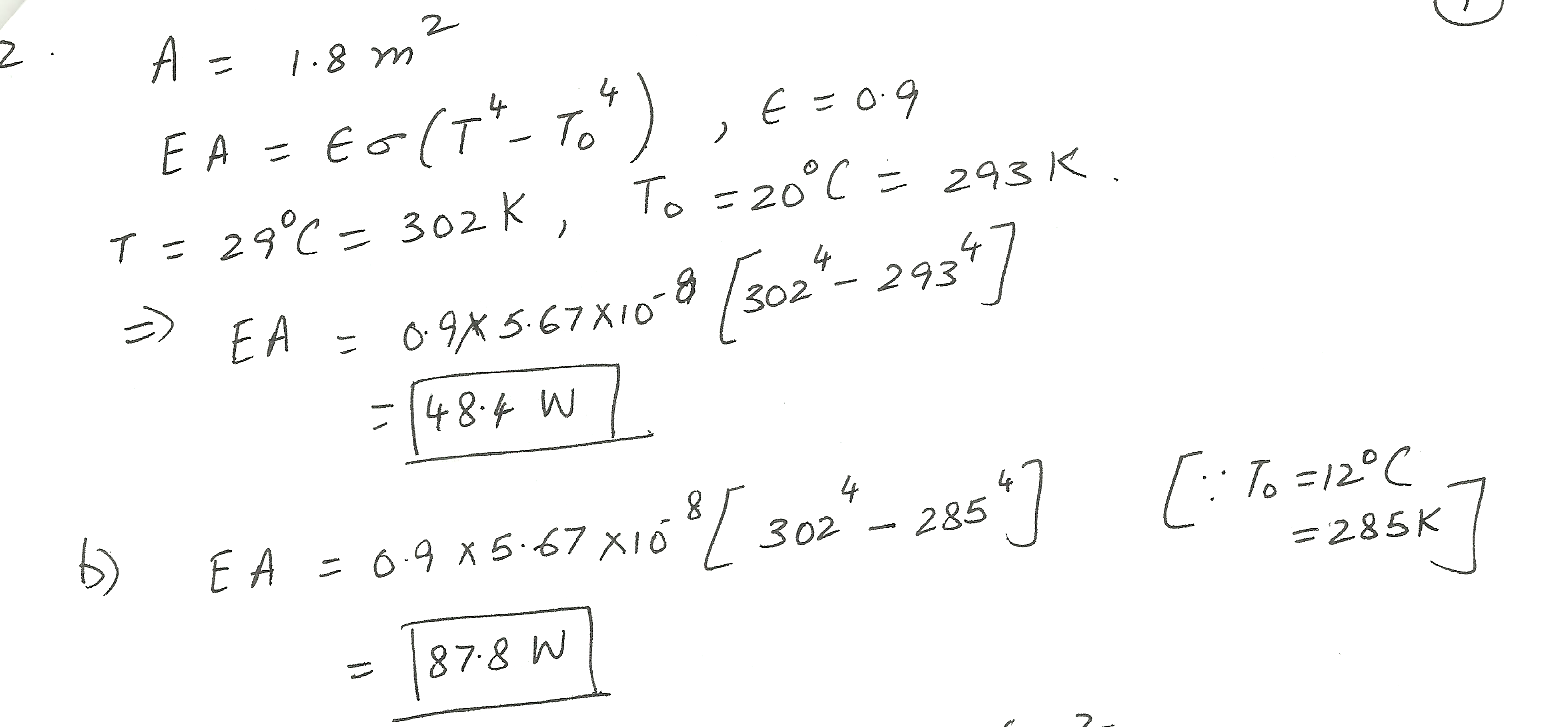
Physics Heat & Thermodynamics Level: Misc Level
A sample of a serum of mass 25 g is cooled from 290 K to 275 K at constant pressure by the extraction of 1.2 k J of energy as heat. Calculate q (energy) and delta H (enthalpy) and estimate the heat capacity of the sample.
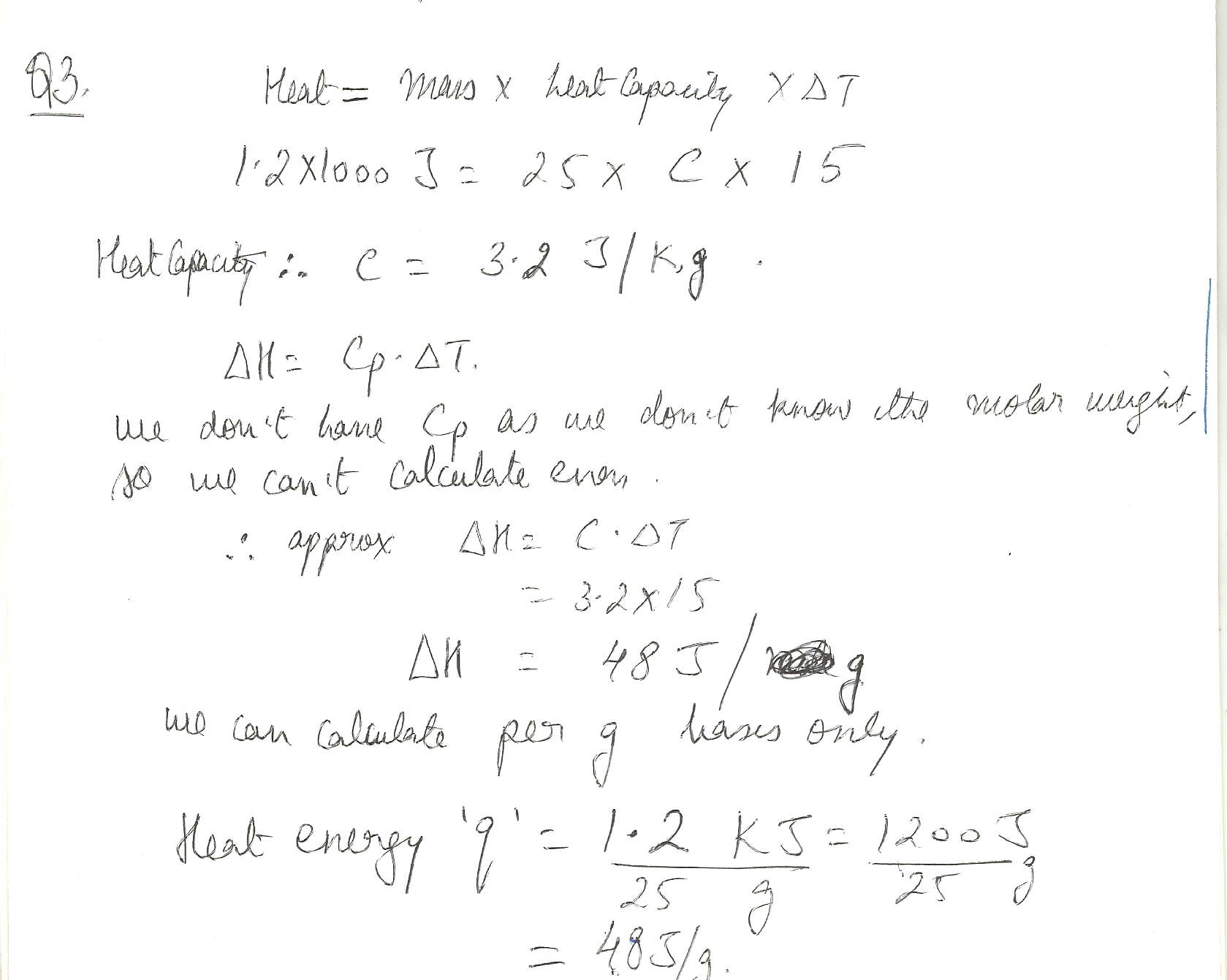
Physics Heat & Thermodynamics Level: Misc Level
How much heat must be removed from 200 g of water initial at a temperature 30 C to convert it completely to ice at 0 C? Assume c water =1.0 kcal / (kg. C )and L f ice =80.0 k cal /kg.

Physics Heat & Thermodynamics Level: Misc Level
A 1 kg sample of wood absorbs 10 kcal of heat and its temperature is found to rise from 20.0C to 44. 0 C.What is the specific heat of the wood?
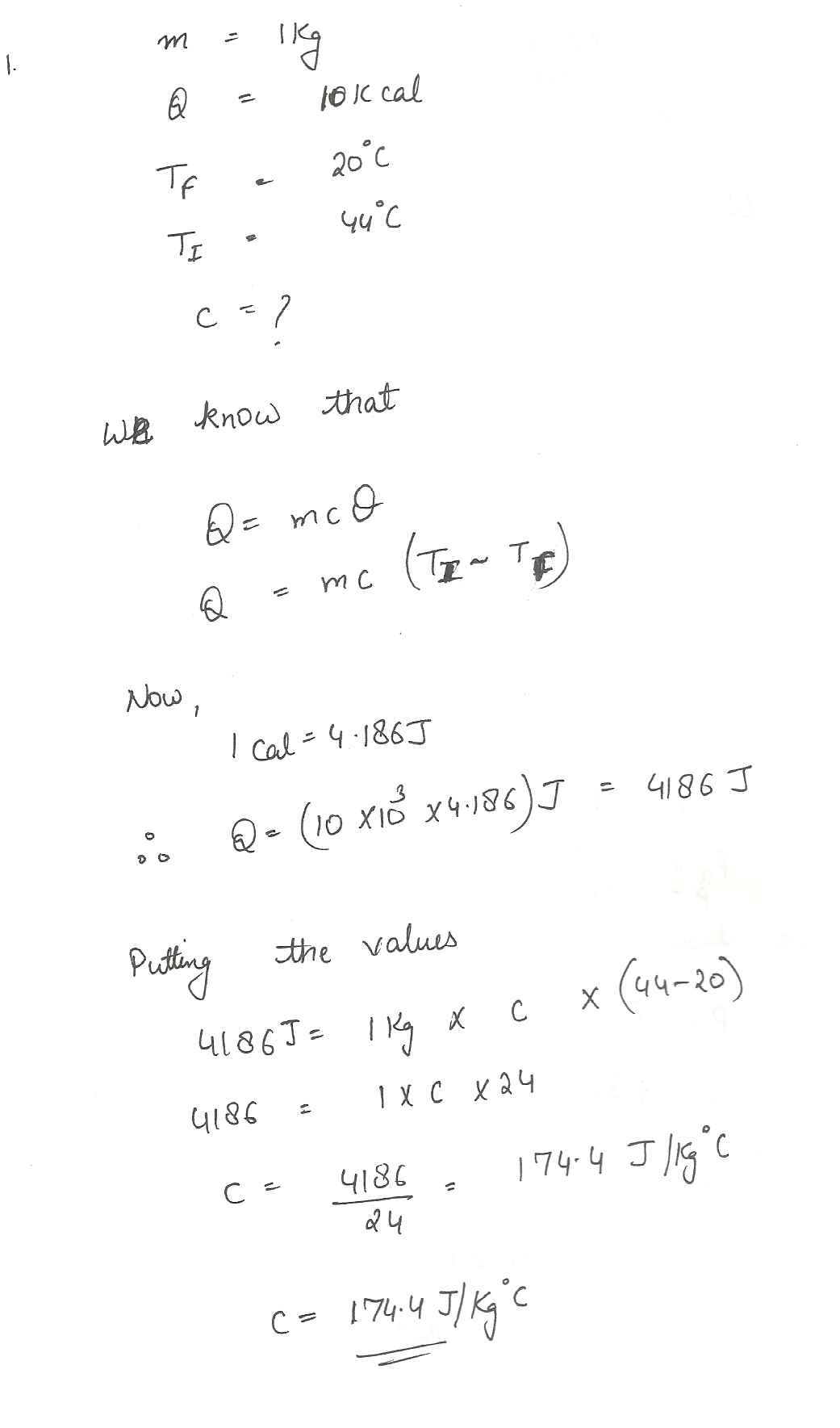
Physics Heat & Thermodynamics Level: Misc Level
A pound of fat contains 3500 Calorie of energy. Jogging burns off 150 Calorie per mile. How far in miles, would you have to jog to burn off 6.00 pounds of fat?Show units in calculations.

Physics Heat & Thermodynamics Level: Misc Level
Calculate the work done by a gas in expanding isothermally from a volume of 1 liter at a pressure of 5 atm to 2 liters.

