Physics Heat & Thermodynamics Level: Misc Level
How many calories of heeded to convert 40 g of ice at a^ C=, 20 C to steam at 200 C at atmospheric pressure.
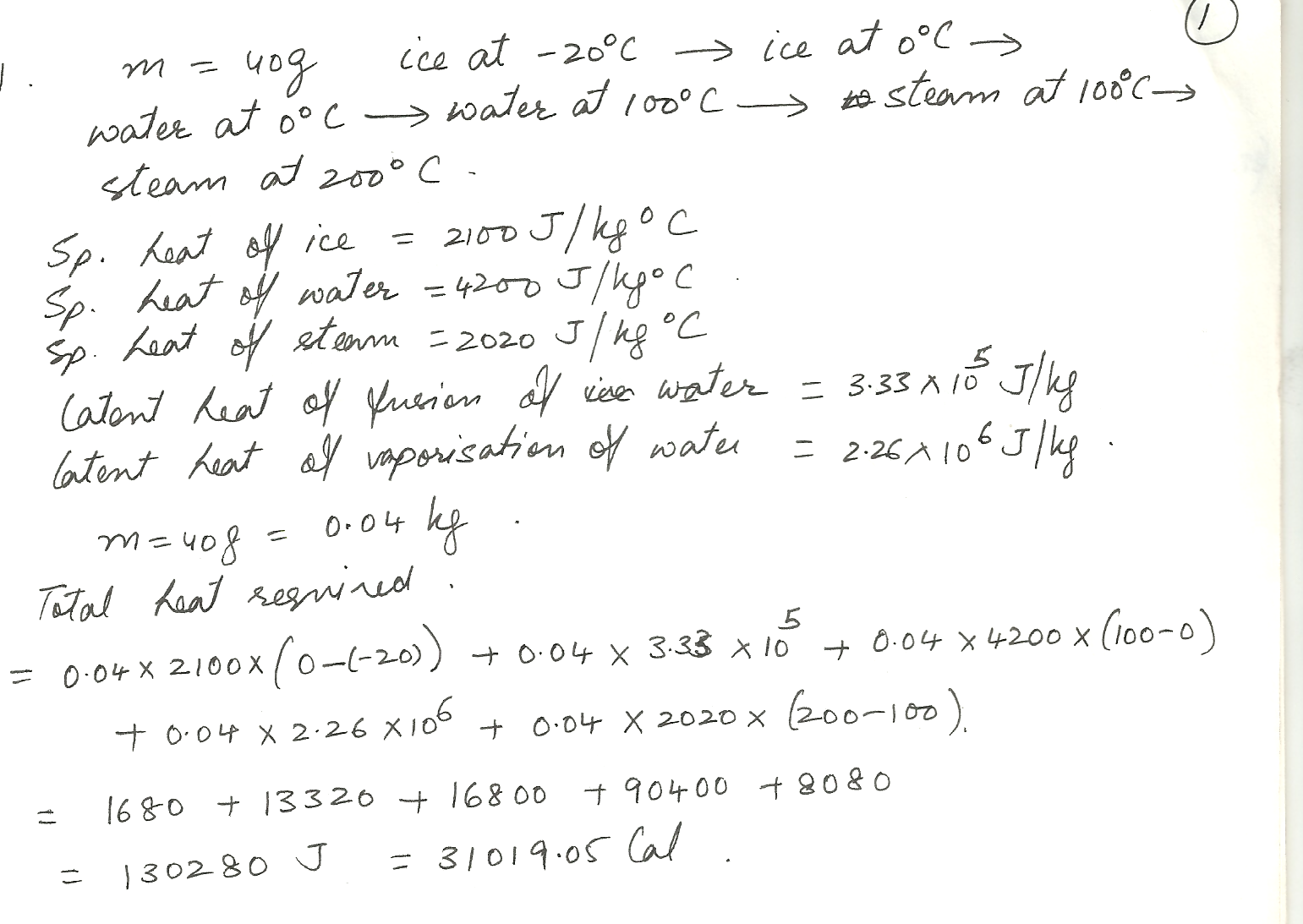
Physics Heat & Thermodynamics Level: Misc Level
An 800 M W electric power plant has an efficiency of 30%.If loses its waste heat in large cooling towers. Approximaterly how much waste heat ( in M J) is discharged to the atmosphere per second?The answer is 1900 M J.

Physics Heat & Thermodynamics Level: Misc Level
Why must a room air conditioner be set partially out of a window? Why can t it just be set in the center of a room and plugged in ? Note that we are talking about an air conditioner, not a water evaporative cooler ( swamp cooler)

Physics Heat & Thermodynamics Level: Misc Level
When an automobile engine overheats and the radiator water begins to boil, the car can still be driven some distance before catastrophic engine damage occurs. Why? What determines the onset of really disastrous overheating?

Physics Heat & Thermodynamics Level: Misc Level
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27 degrees C. (a) If the gas is heated at constant volume untill the pressure triples, what is the final temperature? (b) If the gas is heated so that both the pressure and volume are doubled, what is the final temperature?
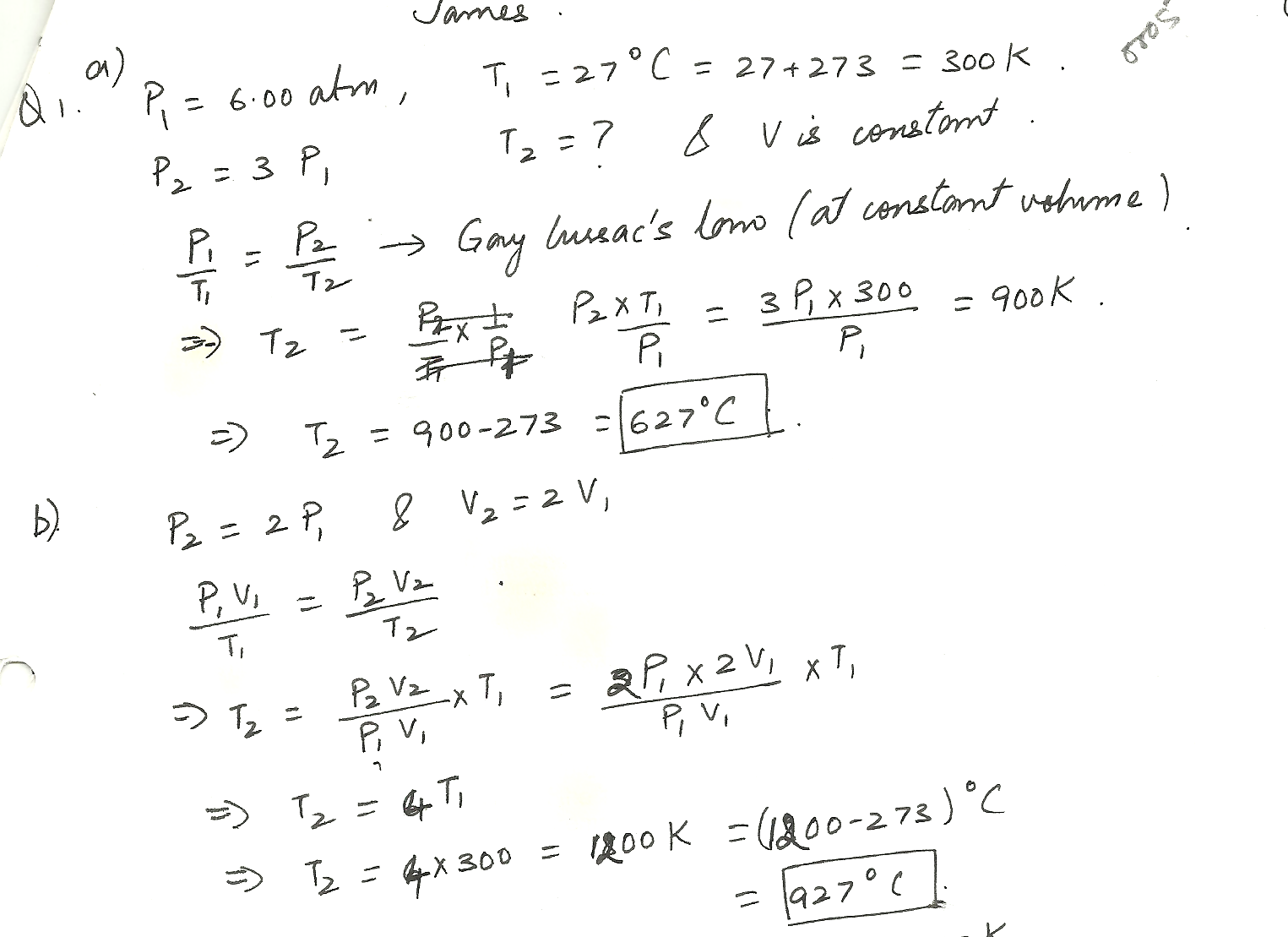
Physics Heat & Thermodynamics Level: Misc Level
Determine the latent heat of vaporization of unknown substance X in kcal /g if 3.0 g of boiling liquid X are completely vaporized in 1.5 hours by an energy source of 10 kcal /h.
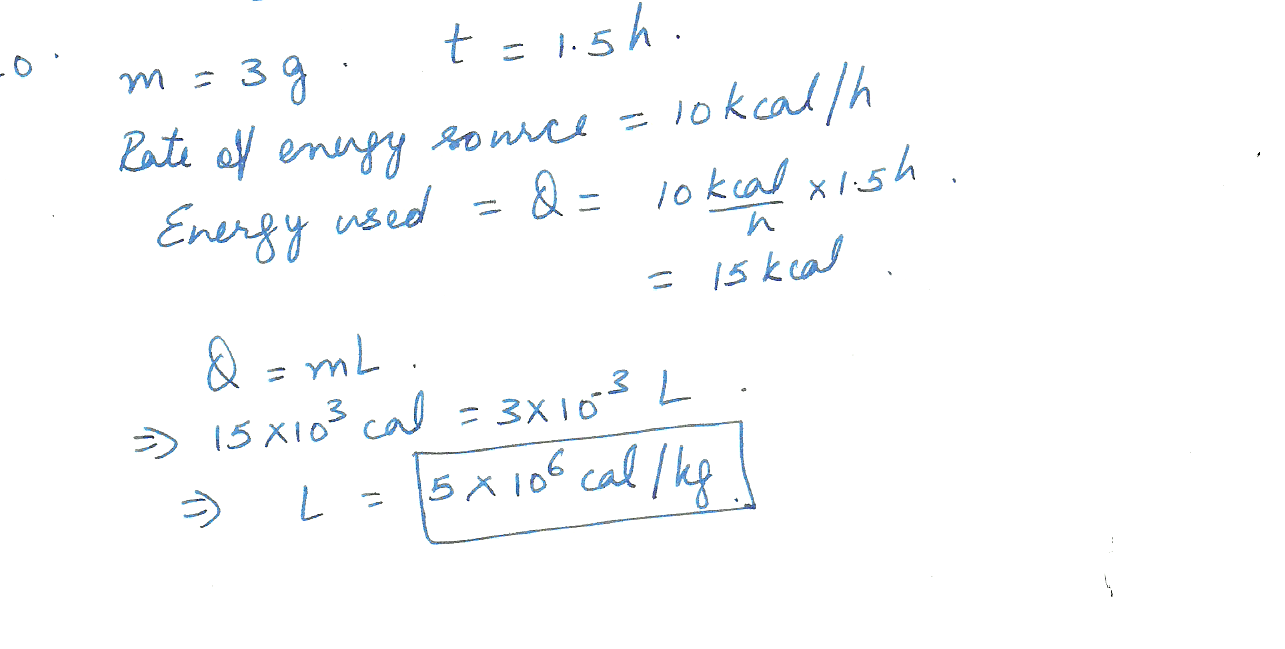
Physics Heat & Thermodynamics Level: Misc Level
Two spheres, labeled A and B, have identical masses, but are made of different substances. The specific heat capacity of sphere A is 440 J /kg C and that of sphere B is 160 J /kg C. There spheres are initially at 21% C: and the same quantity of heat is added to each sphere. If the final temperature of sphere A is 72% C, what is the final temperature of sphere B.?
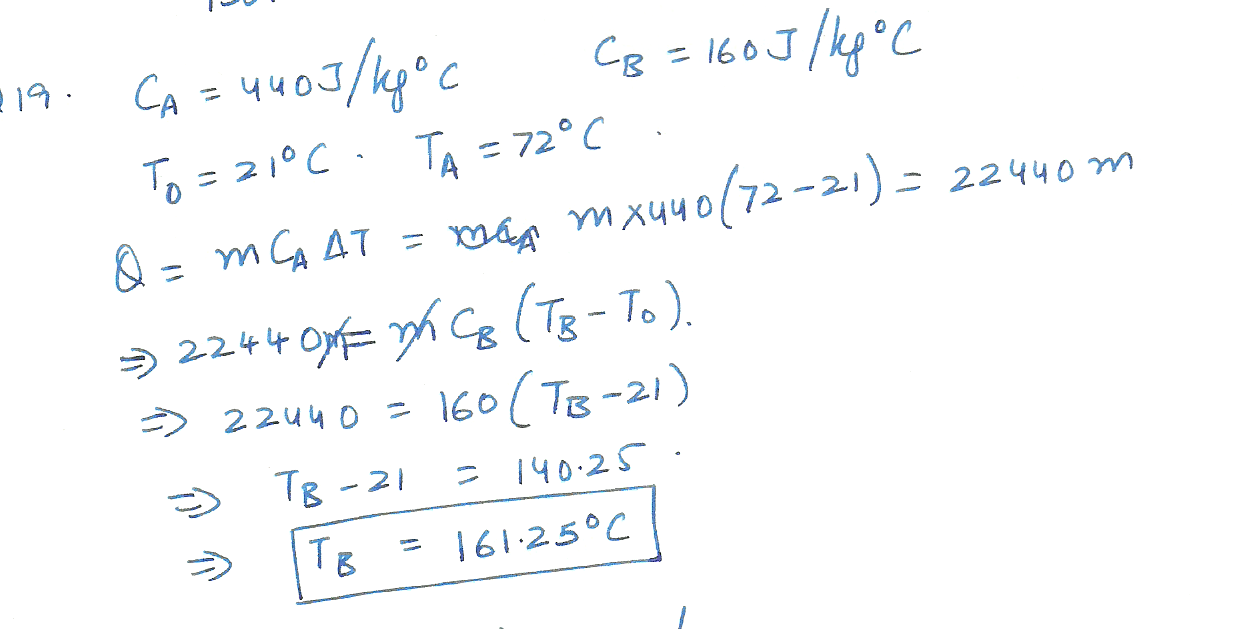
Physics Heat & Thermodynamics Level: Misc Level
Two cubes, one silver and one iron, have the same mass and temperature. A quantity Q of heat is removed from each cube. Which one of the following properties causes the final temperatures of the cubes to be different?
a. Density
b. Latent heat vaporization
c. Specific heat capacity
d. Coefficient of volume expansion
e. Volume
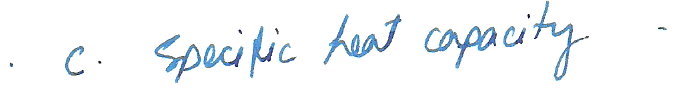
Physics Heat & Thermodynamics Level: Misc Level
How much energy (in J ) is removed from the human body by the evaporation of 9.9 g of water?
Hint: The latent heat of vaporization is 54 kcal /kg for water. Please use only the heat of vaporization, and not the energy required to raise the temperature to 100 %C.
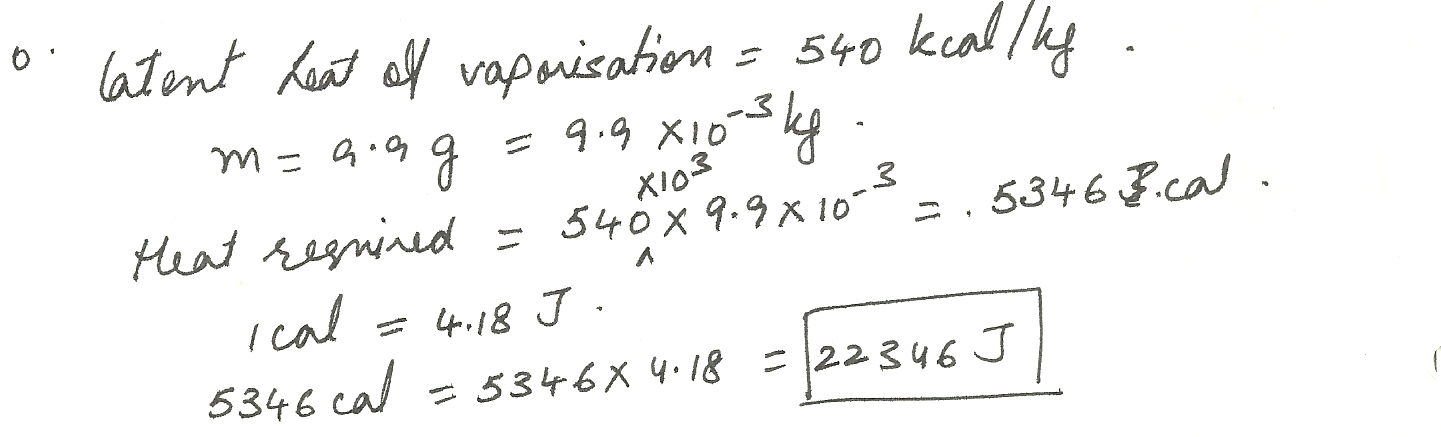
Physics Heat & Thermodynamics Level: Misc Level
If an object has a surface area of 0.7 m2 and an emissivity of 0.61, how much power (in W) does it radiate at a temperature of 34 % C?
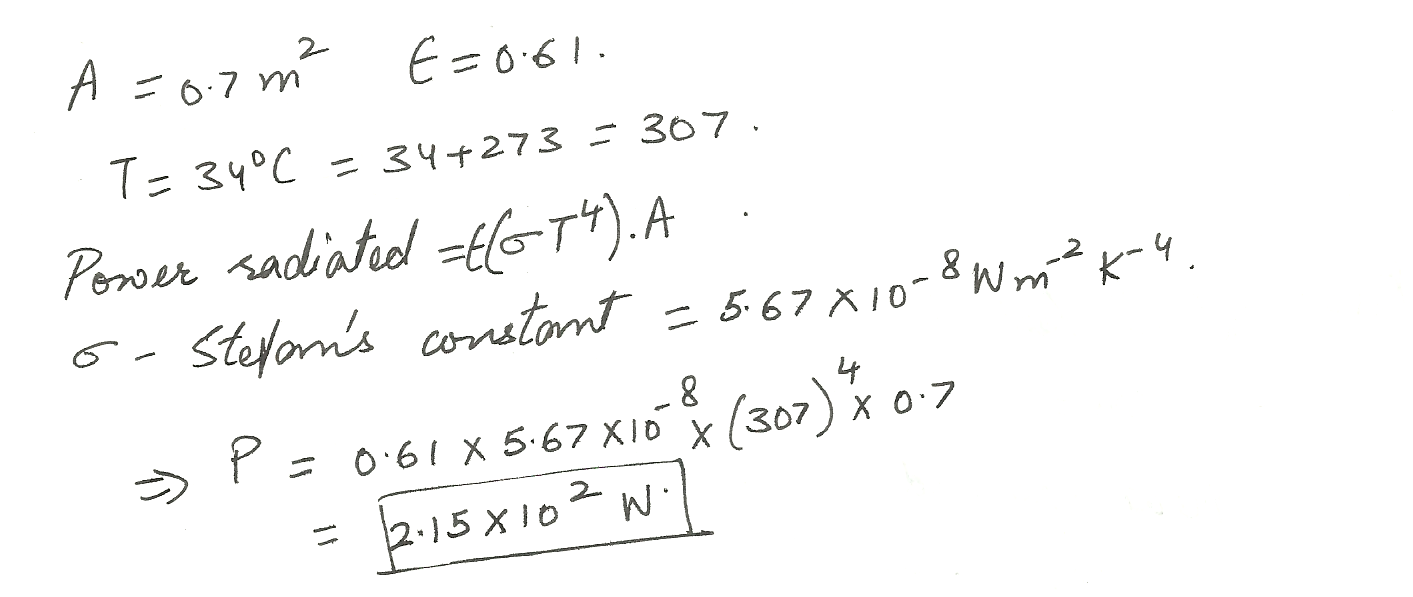
Physics Heat & Thermodynamics Level: Misc Level
How much ( in m) does a concrete slab on the mackinac Bridge 240 m long contract when going from 23 to - 19 degrees % C? The linear expansion coefficient a is 10- 5 per %C.
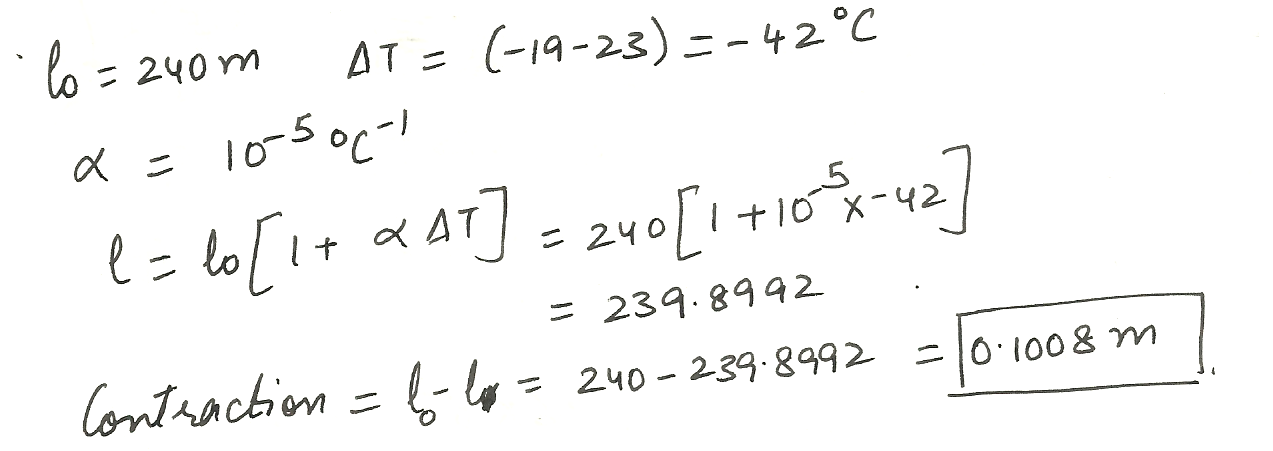
Physics Heat & Thermodynamics Level: Misc Level
A steam engine operates between 550 degrees and 300 degrees C. what is the maximum possible efficiency of this engine?
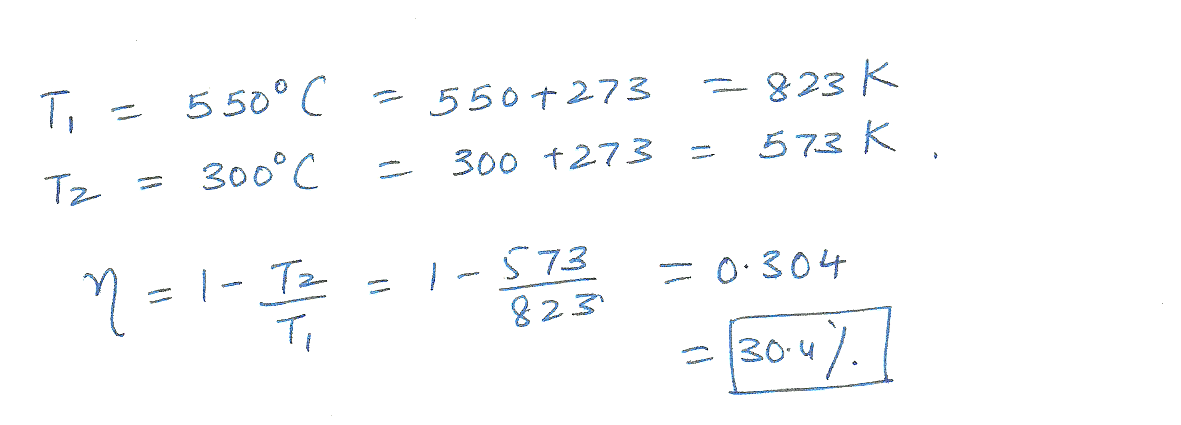
Physics Heat & Thermodynamics Level: Misc Level
In an engine, 0,30 moles of an ideal monatomic gas in the cylinder expands rapidly and adiabatically against the piston In the process, the temperature of the gas drops from 1100 k to 450 k. How much work does the gas contain ?
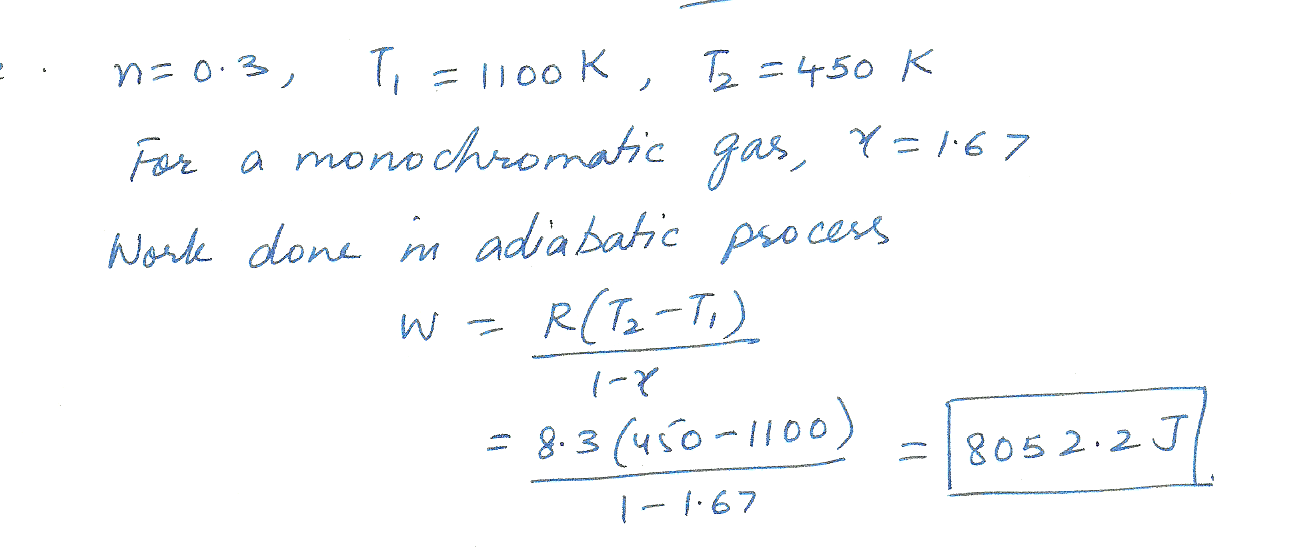
Physics Heat & Thermodynamics Level: Misc Level
A heat engine operates between two reservoirs at temperatures of 20 degrees C and 300 degrees C. what is the maximum efficiency possible for this engine.
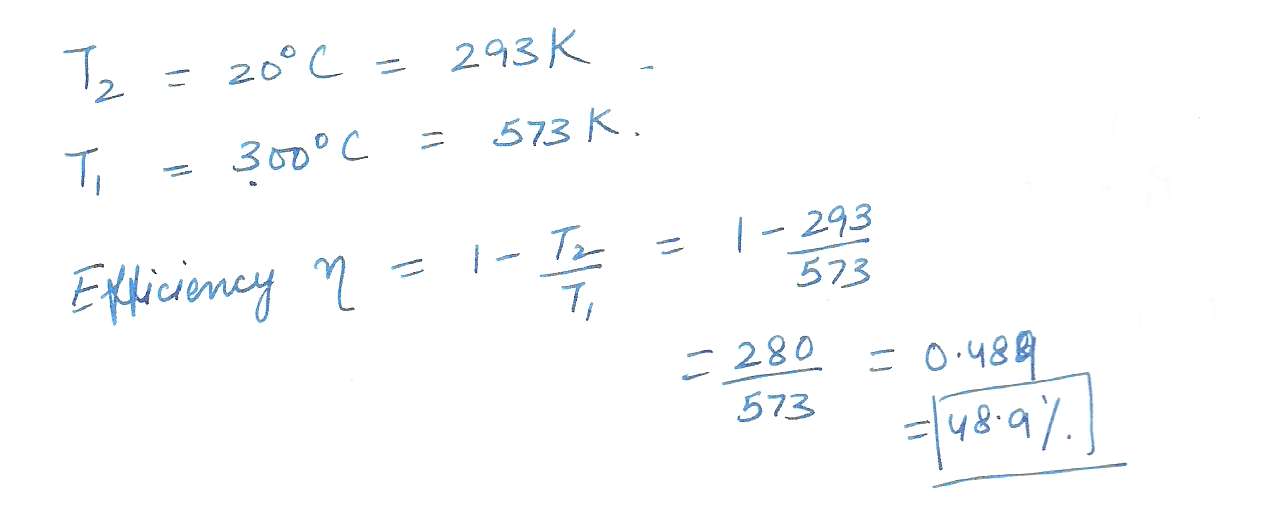
Physics Heat & Thermodynamics Level: Misc Level
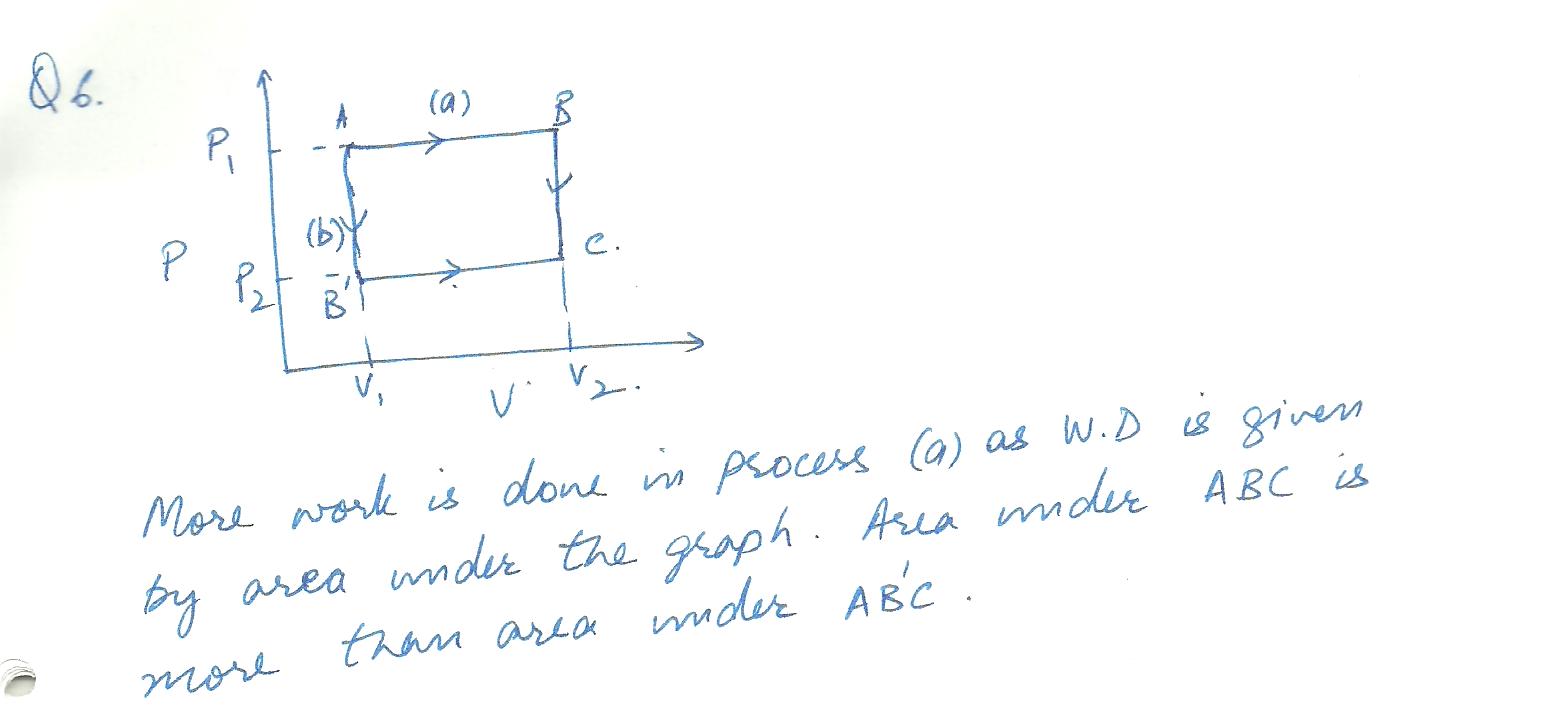
Sketch a PV diagram of the following processes:a A gas expands at constant pressure p1 from volume V 1 to volume V2. It is then kept at constant volume while the pressure is reduced to p2. b. A. gas is reduced in pressure from p1 to p2 while its volume is held constant at v1. It is then expanded at constant pressure p2 to a final volume v2 . c. In which of the processes is more work done by the gas?why?