Physics Heat & Thermodynamics Level: Misc Level
2 grams of ice water at 0 degrees Celsius freezes to ice at 0 degrees Celsius.
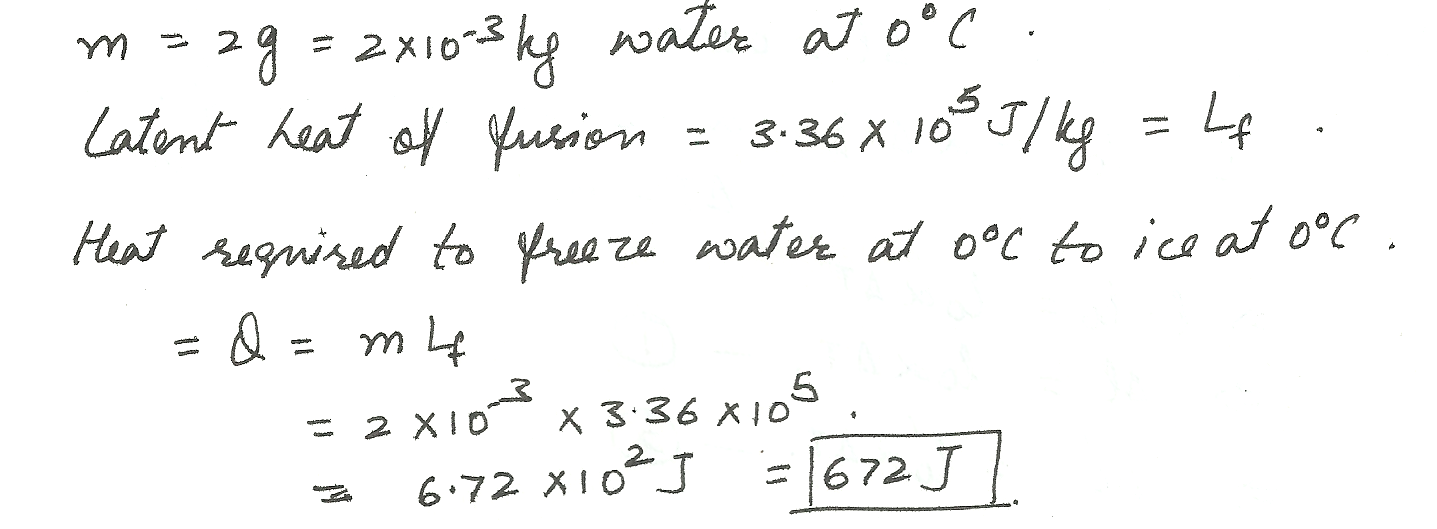
Physics Heat & Thermodynamics Level: Misc Level
2 grams of boiling water at 100 degrees Celsius cools to ice water at 0 degrees Celsius.
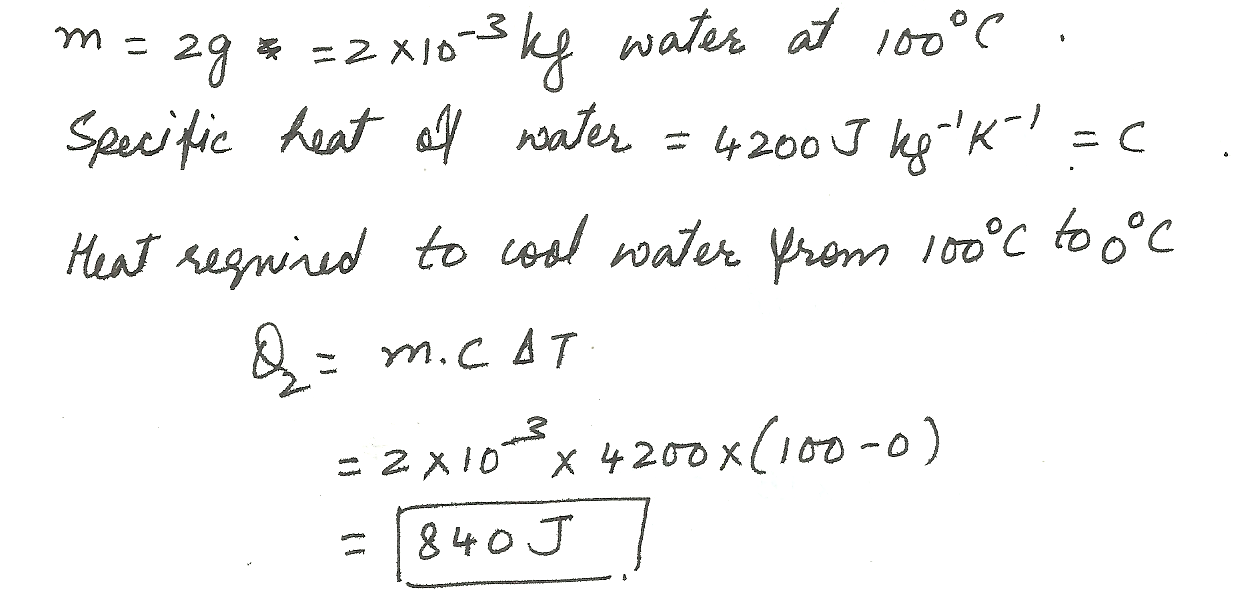
Physics Heat & Thermodynamics Level: Misc Level
2 grams of steam at 100 degrees Celsius condenses to water at 100 degrees Celsius.
Physics Heat & Thermodynamics Level: Misc Level
How many calories are needed to raise 500 grams of water from 25 degrees Celsius to 40 degrees Celsius?
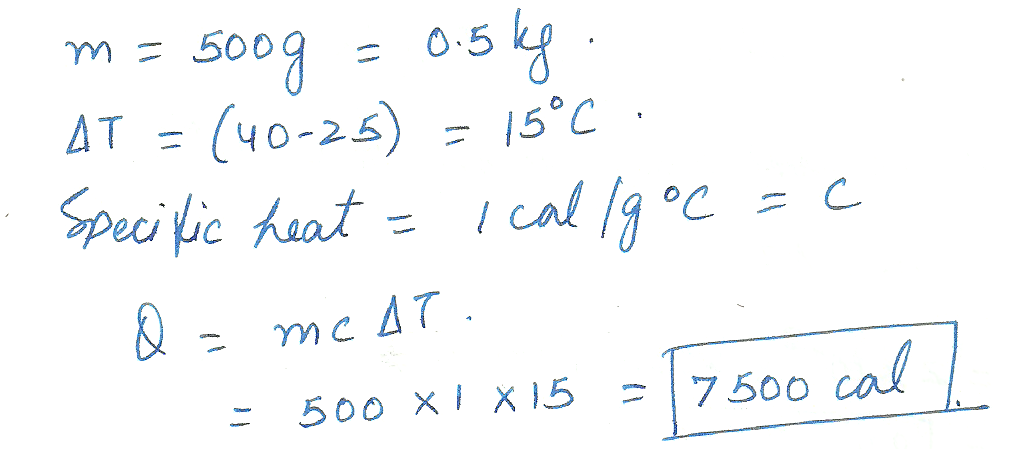
Physics Heat & Thermodynamics Level: Misc Level
An electric motor operates in such a way that is 70 percent efficient when consuming power at the rate of 200 W. The motor is basically copper and has a mass of 0.90 kg Assuming that all the lost power goes into heating the motor, how much will its temperature rise in 1 minute?

Physics Heat & Thermodynamics Level: Misc Level
How many grams of steam at 100 degrees C must be condensed in 500 grams of water at 20 degrees C to raise its temperature to 30 degrees C?

Physics Heat & Thermodynamics Level: Misc Level
How much heat is required to change 50 grams of lead at 20 degrees C to molten lead at 327 degrees C?

Physics Heat & Thermodynamics Level: Misc Level
How much heat must be removed from 50 grams of molten lead at 327 degrees C to change it to a solid?

Physics Heat & Thermodynamics Level: Misc Level
A 90 gram piece of hot iron is dropped into a 500 gram vat of oil at 20 degrees C. The final temperature of the oil and iron is 30 degrees C.Find the original temperature of the iron. (C=0.50 cal/g/degrees C for the oil.)
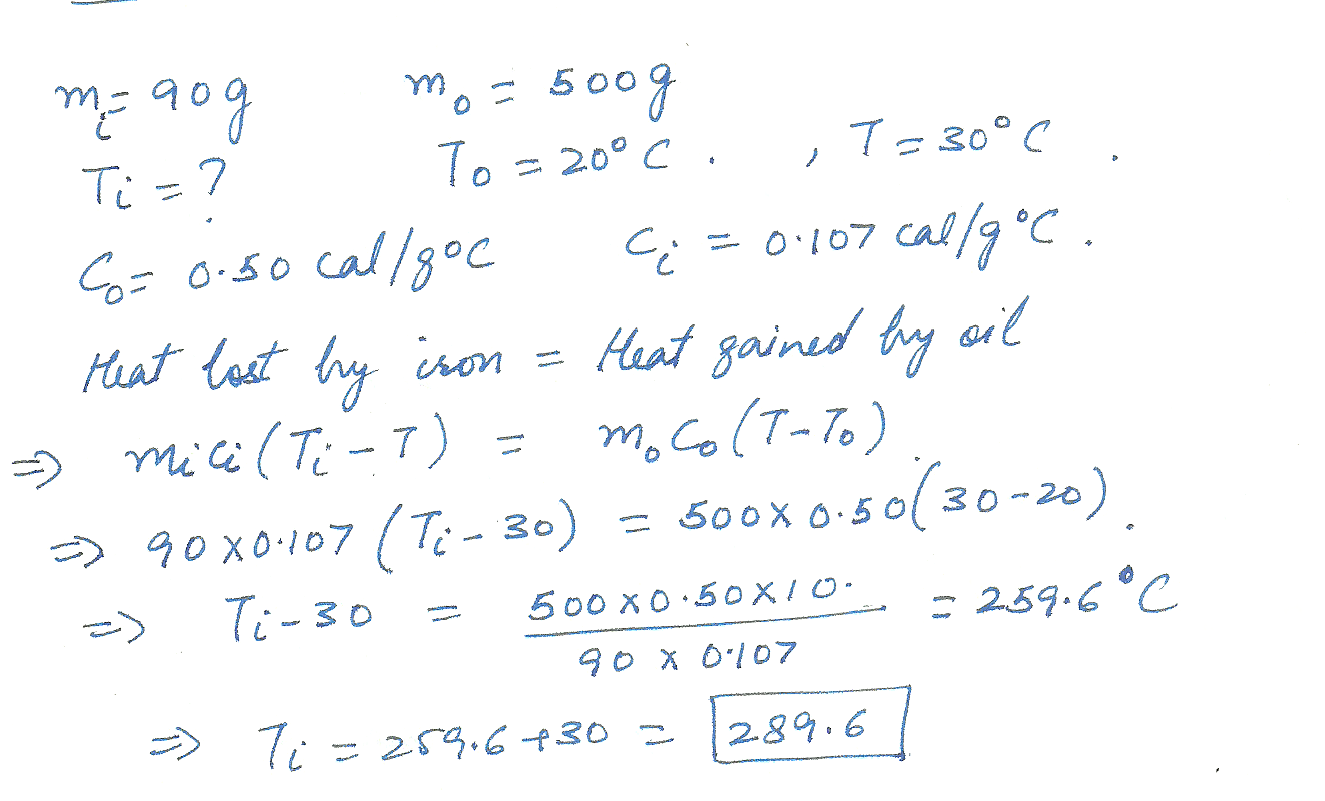
Physics Heat & Thermodynamics Level: Misc Level
A steel ball falls from a height of 20 meters into pile of sand.If one-half its energy ends up as heat in the ball, how much is the ball heated?

Physics Heat & Thermodynamics Level: Misc Level
An experiment to determine the specific heat of a gas makes use of a water manometer attached to a flask (the figure below). Initially the two columns of water are even. Suppose that atmospheric pressure is 1.2 10^5 Pa. After heating the gas, the water level change to those shown. Find the change in pressure of the gas in Pa.
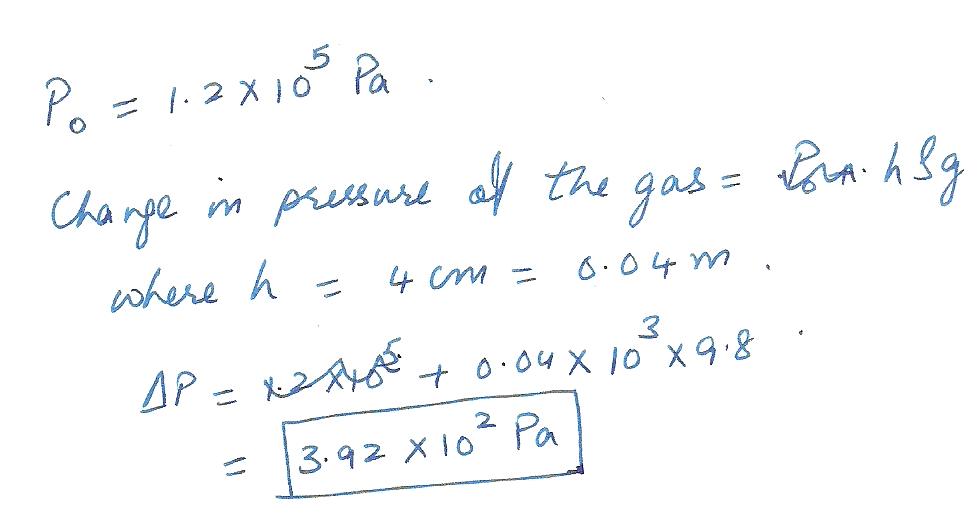
Physics Heat & Thermodynamics Level: University
Charle's LawFind the original temp of a gas in degrees CELSIUS if the gas was moveed from a 75 cubic centimeter tank to a 225 cubic centimeter tank with a temperature of 123 degrees Celsius.
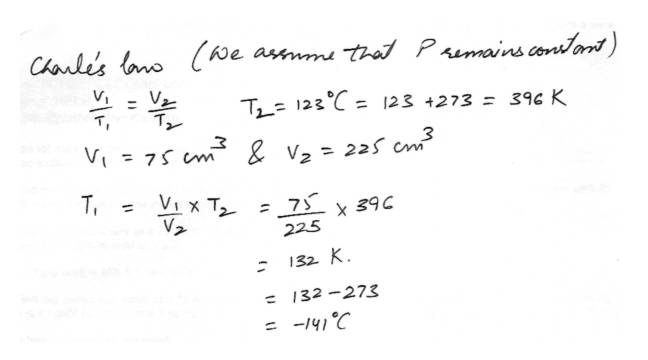
Physics Heat & Thermodynamics Level: University
ExpansionAt 20.0 degrees celius a copper disk is 10.00cm in diameter and a hole in the disk is 0.4000 cm in diameter. What are the diameters of the disk and the hole at 200 degress celius? The coefficient of the linear expansion of copper is 1.70 x 10^-5/ ( degrees celius)

Physics Heat & Thermodynamics Level: University
GasA gas contains molecules made with two different atoms. Measurements of the specific heat give results as stated. From T(sub-1) to T (sub-2) Cv/R = 1.5 from Describe the value of the three different results for Cv in the given temperature ranges as well as you are able to.

Physics Heat & Thermodynamics Level: University
SpecificheatA small meteorite comprised of iron with mass 1.32 x 10^3 kg enters Earths atmosphere from space and crashes into lake camplain ( a body of fresh water which has a total mass of 8.56 x 10^8 kg). The entry into Earths atmosphere increases the temperature of the meteorite to 3.24 x 10^3 degrees celius just before it comes apart in small pieces and impacts he water at a speed of 3500 m/s above the deepest part of the lake. The temperature of the lake is a chilly 10 degrees celius just before the impact. What is the final temperature of the system of the lake and meteorite? For this purpose assume the lake bottom is a good thermal insulator.

