Physics Heat & Thermodynamics Level: University
Kinetic Theory of gasWhy dont the sizes of different molecules present in a gas enter into the equation for the gas laws?

Physics Heat & Thermodynamics Level: University
Thermal ExpansionIf your property must be measured with a metal tape in order to determine the amount of your property taxes would you rather have it measured in the summer or the winter? Remember that your property will not significantly expand or contract with change in season.
.
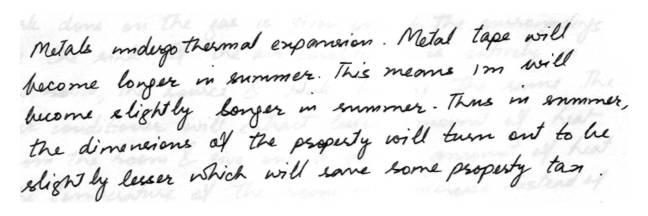
Physics Heat & Thermodynamics Level: University
Units conversion of energyA soft drink manufacturer claims that a new drink is low joule. The label indicates the available energy is6300 J. What is the equivalent of this energy in calories ( 1 Calorie = 1000 cal)?
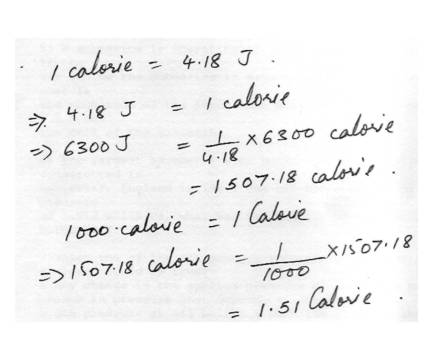
Physics Heat & Thermodynamics Level: University
VolumetricZirconium tungstate is an unsual material because its volume shrinks with an increase in temperature for the temperature range 0.3 K to 1050 K(where it decomposes). In fact the volumetric coefficient of thermal expansion is - 26.4 x 10 ~6/k. Determine the ratio ( delta V/final V) for the above mentioned temperature range. Express your answer in percent.

Physics Heat & Thermodynamics Level: University
ExpansionA metal rod 40.0000-cm long at 40oC is heated to 60oC. The length of the rod is then measured to be 40.0105-cm. What is the coefficient of linear expansion of the metal in units of /oC?

Physics Heat & Thermodynamics Level: University
Ideal Gas EquationA volume of 50.0 liters of 10 degree C and an absolute pressure of 1.88 atm is compressed to 36.6 liters and at the same time the temperature is raised to 80 degree C. What will the absolute pressure be now?
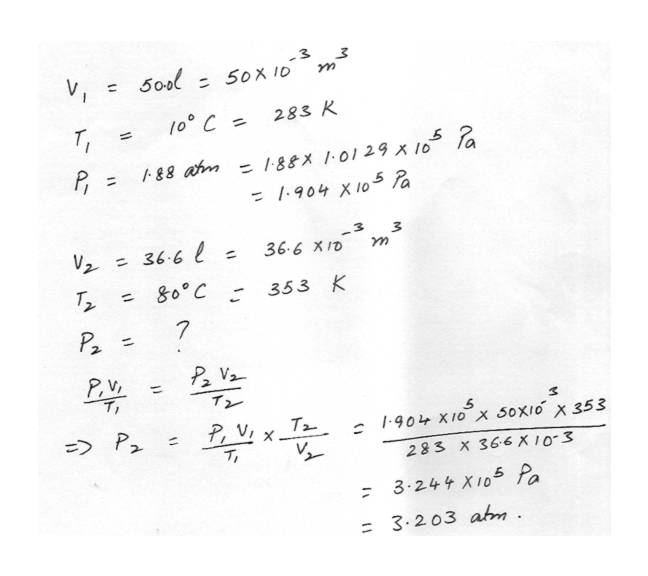
Physics Heat & Thermodynamics Level: University
Ideal Gas EquationA volume of 50.0 liters of oxygen at 100C and an absolute pressure of 1.88 atm is compressed to 36.6 liters and at same time the temperature is raised to 800C. What will the absolute pressure be now?
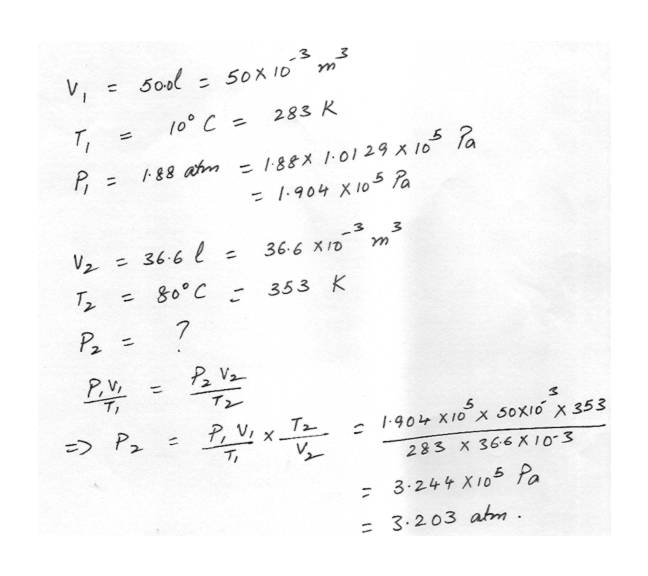
Physics Heat & Thermodynamics Level: University
Principle of calorimetryCalculate the latent heat of fusion of ice from the following data for ice at 0 degree C added to water in a calorimeter. ( Give your result to three significant figures)
specific heat of the calorimeter 0.100 cal (gm degree C)
Mass of the calorimeter 70.0 gm
Mass of the calorimeter plus water 507.0 gm
Mass of ice added 169.0 gm
Initial temperature of water plus calorimeter 40.0 degree C
Final Temperature of the mixture 7.0 degree C

Physics Heat & Thermodynamics Level: University
Efficiency of Carnot engineAn engine operates at half its theoretical (Carnot) efficiency between 545 degree C and 310 degree C. What is the efficiency of the engine?
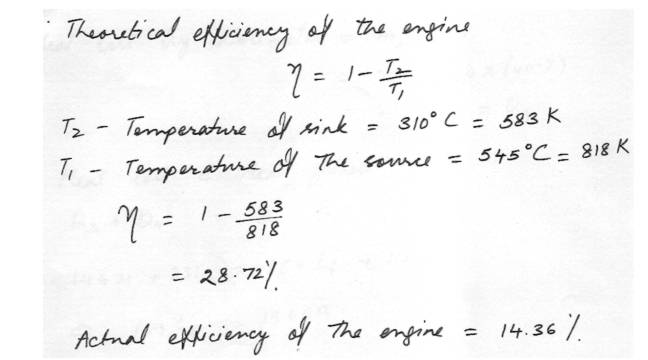
Physics Heat & Thermodynamics Level: University
Thermal expansionA steel 115 mm long has a cross-sectional area of 145 mm^2. When its temperature is increased by 25 degree C it will expand ( lengthen) by a certain small amount delta L. What stretching force F would need to be applied to this same bar to cause this same change in length ( delta L) without any change in temperature? For steel Y = 20 x 10^10 N/m^2

Physics Heat & Thermodynamics Level: University
Thermodynamic processAn adiabatic process is one in which the system being considered:
a. Remains at contant temperature
b.Remains at constant pressure
c. Has no heat flow into or out of it during the process.
d. Undergoes no change in internal energy.
e. Does no work and has no work done on it.
Physics Heat & Thermodynamics Level: University
EntropyA 1-kg chunk of ice at 0 degree C melts absorbing 80000 cal of heat in to process. Which of the following best describes what happens to this system?
a. Increased entropy
b.Lost entropy
c. Entropy maintained constant.
d. Work converted to energy.
Physics Heat & Thermodynamics Level: University
Kinetic theory of GasThe absolute temperature of an ideal gas is directly proportional to which of the following properties ( when taken as an average) of the molecules of that gas?
a. Speed
b. Momentum
c. Mass
d. kinetic energy
Physics Heat & Thermodynamics Level: University
In a cloud formation water vapor turns into water droplets which get bigger and bigger until it rains. This will cause the temperature of the air in the clouds to:a. Get warmer
b. Get cooler
c. Be completely unaffected
d.There is no air in clouds
Physics Heat & Thermodynamics Level: University
Transfer of heatWhich of the following processes of heat transfer may take place in a vacuum?
a. Conduction
b. Convection
c.Radiation
d. Induction
e. None of the above choices are valid
