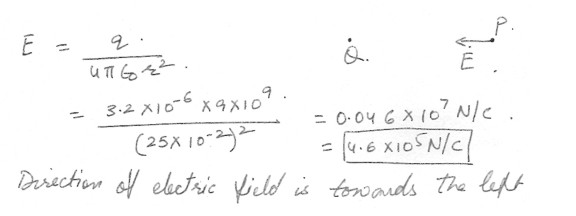Physics Heat & Thermodynamics Level: University
GasHow much force does the atmosphere exert on one side of a vertical wall 4.0 m high and 10 m long?

Physics Heat & Thermodynamics Level: University
GasThe average density of the material in intergalactic space is approx. 2.5 x 10 - 27 kg/m3. What is the volume of gold sample p = 19300 kg/m3 that has the same mass as 8.0 x 1024 m3 of intergalactic space?

Physics Heat & Thermodynamics Level: University
GasAt standard temperature and pressure carbon dioxide has a density of 1.98 kg/m3. What volume does 0.85 kg of carbon dioxide occupy at standard temperature and pressure?

Physics Heat & Thermodynamics Level: University
A rotating 5 kg solid flywheel ( disk) has a radius of 50 cm and an angular velocity of 20 rads/ second. The wheel is brought to rest and all of the rotational energy of the flywheel goes into heating a surrounding water bath. If the water temperature increases 0.2 degrees celcius what is the mass of the water surrounding the flywheel?
Physics Heat & Thermodynamics Level: University
Steam at 100oC is added to ice at 0oC. (a) find the amount of ice melted and the final temperature when the mass of steam is 10g and the mass of ice is 50g (b) repeat with steam of 1.0g and ice of mass 50g.
Physics Heat & Thermodynamics Level: University
A 75kg cross country skier glides over snow. The coefficient of friction between skis and snow is 0.20. Assume all the snow beneath his skis is at 0oC and that all the internal energy generated by friction is added to snow that sticks to his skis until melted. How far would he have to ski to melt 1.0 kg of snow?
Physics Heat & Thermodynamics Level: University
Heat Energy TransferA single food calorie is enough energy to raise 1.0kg how high?
a. 43 cm
b. 72 cm
c. 0.43 km
d. 0.72 km
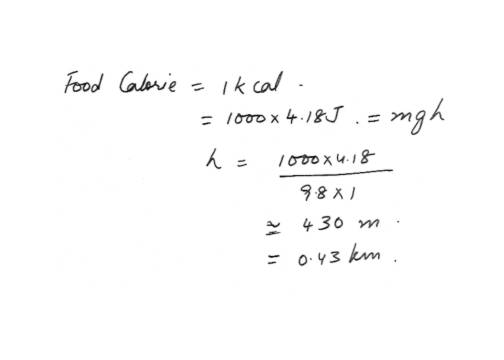
Physics Heat & Thermodynamics Level: High School
A 50.0 g lump of metal at temperature 151 degrees C is placed in a calorimeter cup containing 30.0 grams of water at 23 degrees C. After equilibrium is established, the temperature is 49 degrees C. The heat capacity of the cup is 42 J/K. What is the specific heat cm of the metal?
Physics Heat & Thermodynamics Level: High School
A weight lifter manages to snatch a 104 kg weight a distance of 1.5 m . How many kcal of heat energy are equivalent to this lift ?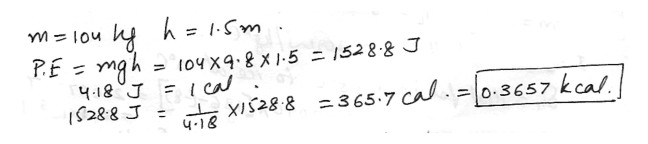
Physics Heat & Thermodynamics Level: High School
How much energy (in J) is removed from the human body by the evaporation of 9.9 g of water ?
(Hint : The latent heat of vaporization is 540 kcal/kg for water. Please use only the heat of vaporization, and not the energy required to raise the temperature to 100 degree C .
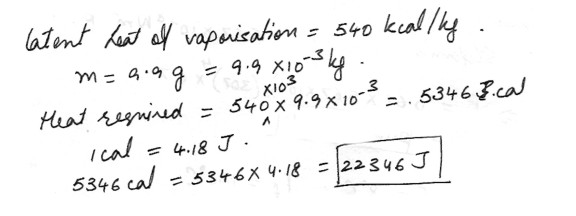
Physics Heat & Thermodynamics Level: High School
Change of state
Heat is added to 0.7 kg of ice at -8 degree C . How many kilocalories are required to change ice to steam at 136 degree C .

Physics Heat & Thermodynamics Level: High School
Radiation
If an object has a surface area of 0.7 m2 and an emissivity of 0.61 , how much power (in W) does it radiate at a temperature of 34 degree C ?
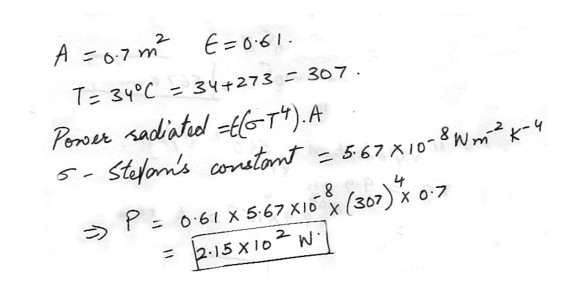
Physics Heat & Thermodynamics Level: High School
Thermal expansion
A can of gasoline has a rectangular base with dimensions of 13 cm by 10 cm . If there is 0.5 liters of gasoline in the can . how much does the surface of the gasoline rise (in mm) in the can when the temperature is raised by 34 degree C ? The coefficient for volume expansion of gasoline is 9.5 10-4/ degrees C .
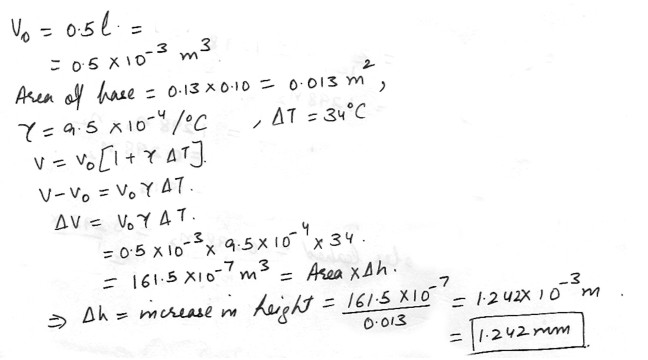
Physics Heat & Thermodynamics Level: High School
Thermal expansion
How much (in m ) does a concrete slab on the Mackinac Bridge 240 m long contract when going from 23 to -19 degrees 0C ? The linear expansion coefficient a is 10-5 per degree 0C ?
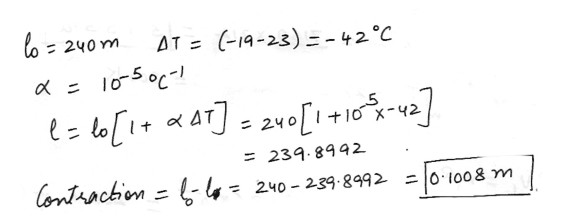
Physics Heat & Thermodynamics Level: High School
Calculate the magnitude and direction of the electrical field at a point P which is 25 cm to the right of a point charge Q = -3.2 x 10 to the -6 C .