Physics Heat & Thermodynamics Level: High School
Ideal Gas Equation
a) An ideal gas occupies a volume of 2.8 cm^3 at 20 degree C and atmospheric pressure . Determine the number of molecules of gas in the container .
b) If the pressure is reduced to 2.6 x 10^-11 Pa ( an extremely good vacuum) while the temperature remains constant , how many moles of gas remain in the container ?
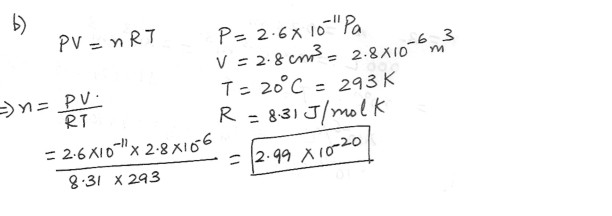
Physics Heat & Thermodynamics Level: High School
Thermal Expansion
A hollow aluminum cylinder 23.0 cm deep has an internal capacity of 2.00 L at 20.0 degree C . It is completely filled with turpentine and then warmed to 74.0 degree C .a) How much turpentine overflows ?
b) If it is then cooled back to 20.0 degree C , how far below the surface of the cylinder’s rim is the turpentine’s surface ?

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
On a day when the temperature is 20.5 degree C , a concrete walk is poured in such a way that its ends are unable to move .a) What is the stress in the cement when its temperature is 49.5 degree C ?
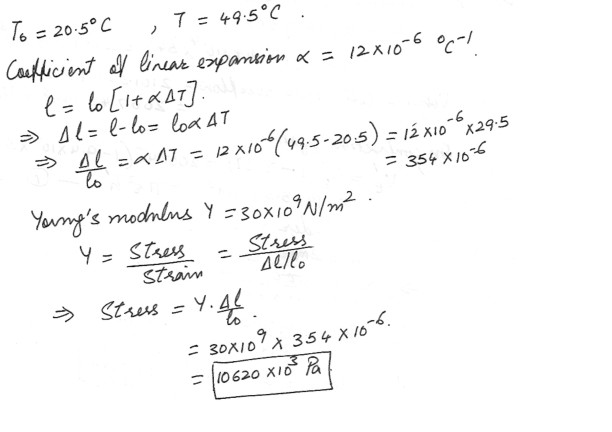
Physics Heat & Thermodynamics Level: High School
Thermal Expansion
The average coefficient of volume expansion for carbon tetrachloride is 5.81 x 10^-4 (degree C)^-1 . If a 49.5 gal steel container is filled completely with carbon tetrachloride when the temperature is 10.0 degree C , how much will spill over when the temperature rises to 30.0 degree C ?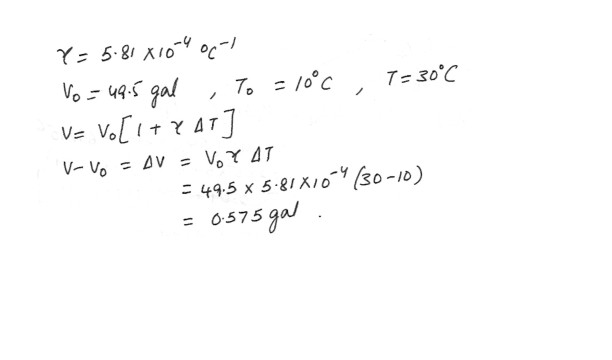
Physics Heat & Thermodynamics Level: High School
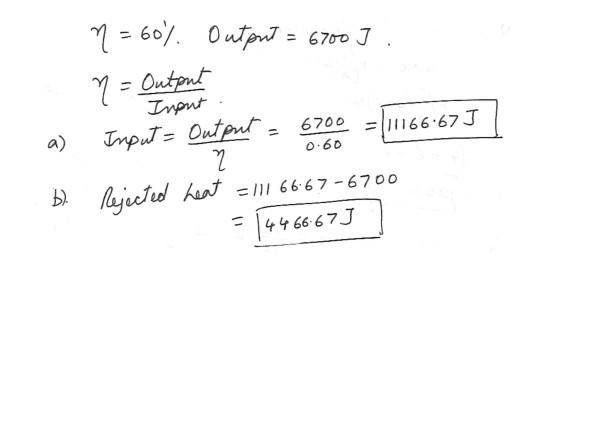
Heat Engine
An engine has an efficiency of 60% and produces 6700 J of work . Determine each of the following .a) the input heat
b) the rejected heat
Physics Heat & Thermodynamics Level: High School
First Law of Motion
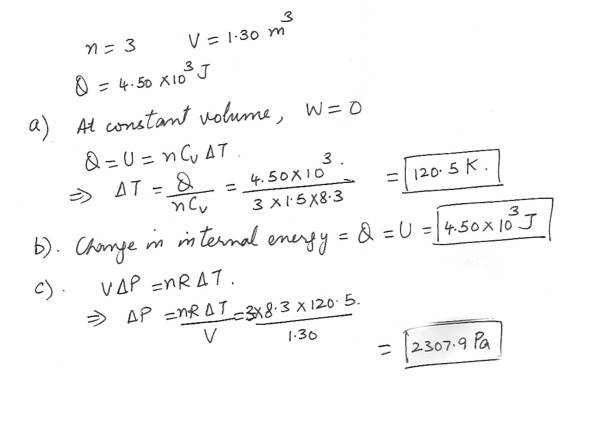
Three moles of a monatomic ideal gas are heated at a constant volume of 1.30 m^3 . The amount of heat added is 4.50 x 10^3 J .a) What is the change in the temperature of the gas ?
b) Find the change in its internal energy ?
c) Determine the change in pressure .
Physics Heat & Thermodynamics Level: High School
Work done from P.V graph
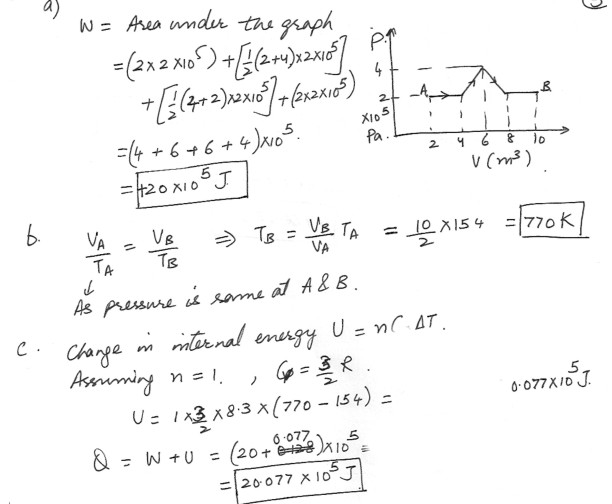
A monatomic ideal gas expands from point A to point B along the path shown in the drawing which is shown at “click here”a) Determine the work done by the gas .
b) The temperature of the gas at point A is 154 K . What is its temperature at point B ?
c) How much heat has been added to the gas during the process ?
Physics Heat & Thermodynamics Level: High School
Work done in adiabatic expansion
Two moles of a monatomic ideal gas expand adiabatically, and its temperature decreases from 370 to 170 K .a) What is the work done by (or done to) the gas ? Including the algebraic sign .
b) What is the change in the internal energy of the gas ? Include the algebraic sign .

Physics Heat & Thermodynamics Level: High School
First Law of Thermodynamics
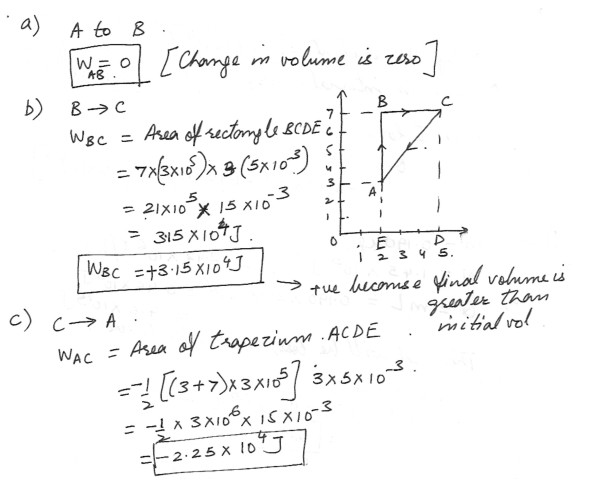
The pressure and volume of a gas are changed along a path ABCA in the figure which is shown at “click here” . The vertical divisions on the graph represent 3.00 x 10^5 Pa , and the horizontal divisions represent 5.00 x 10^-3 m^3 . Determine the work done (including algebraic sign) in each segment of the path .a) A to B
b) B to C
c) C to A
Physics Heat & Thermodynamics Level: High School
A gas , while expanding under isobaric conditions , does 420 J of work . The pressure of the gas is 1.6 x 10^5 Pa , and its initial volume is 1.60 x 10^-3 m^3 . What is the final volume of the gas ?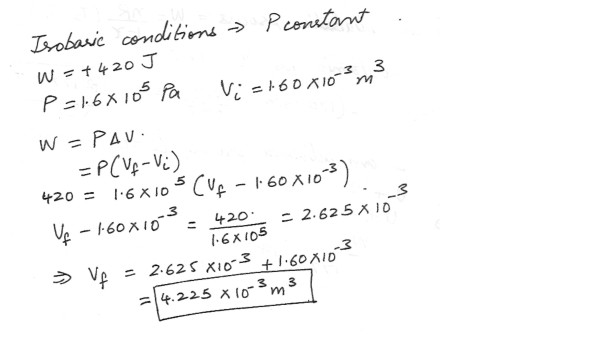
Physics Heat & Thermodynamics Level: High School
First Law of Thermodynamics
In exercising , a weight lifter loses 0.190 kg of water through evaporation , the heat required to evaporate the water coming from the weight lifter’s body . The work done in lifting weights is 1.45 x 10^5 Ja) Assuming that the latent heat of vaporization of perspiration is 2.42 x 10^6 J/kg , find the change in the internal energy of the weight lifter .
b) Determine the minimum number of nutritional calories of food (1 nutritional calorie = 4186 J ) that must be consumed to replace the loss of internal energy .
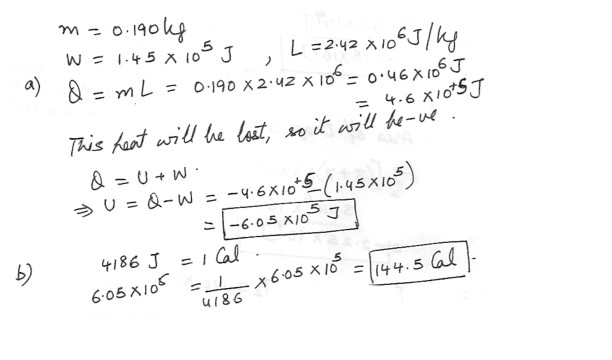
Physics Heat & Thermodynamics Level: High School
First Law of Thermodynamics
In moving out of a dormitory at the end of the semester , a student does 1.90 x 10^4 J of work , In the process , his internal energy decreases by 3.90 x 10^4 . Determine W,U , and Q (including the algebraic sign) .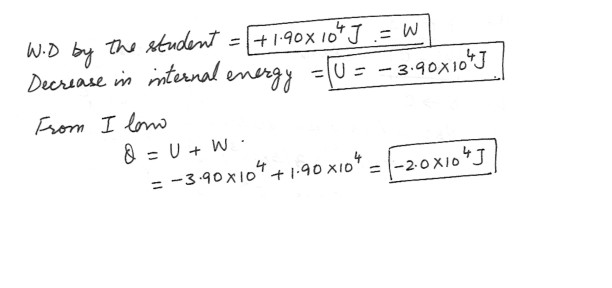
Physics Heat & Thermodynamics Level: High School
Entropy
An aluminum rod conducts 7.79 cal/s from a heat source maintained at 252 degree C to a large body of water at 28.8 degree C . Calculate the rate entropy increases per unit time in this process .
Physics Heat & Thermodynamics Level: High School
Refrigerator
A restaurant refrigerator has a coefficient of performance of 5.12 . If the temperature in the kitchen outside the refrigerator is 30.1 degree C , what is the lowest temperature (in Celsius ) that could be obtained inside the refrigerator if it were ideal ?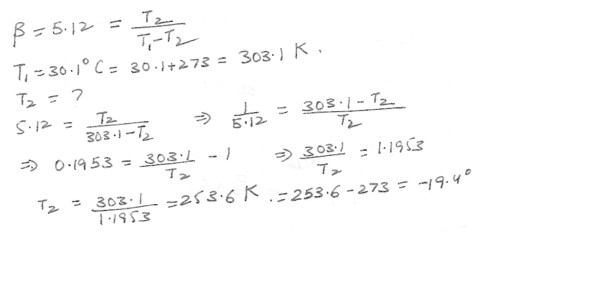
Physics Heat & Thermodynamics Level: High School
Carnot engine
A Carnot engine performs work at the rate of 426 kW while using 636 kcal of heat per second . If the temperature of the heat source is 525 degree C , at what temperature (in Celsius) is the waste heat exhausted ?
