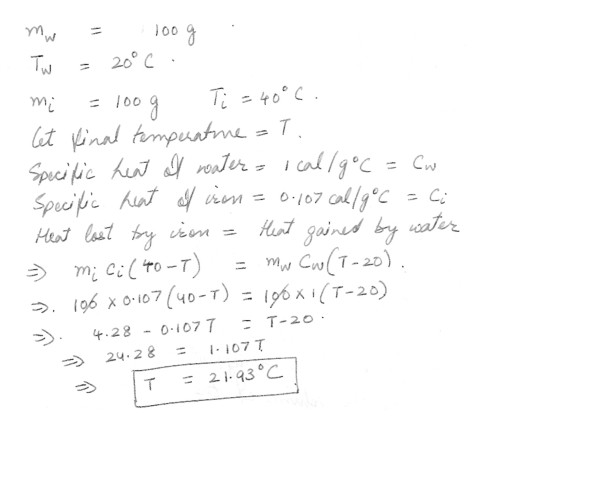Physics Heat & Thermodynamics Level: High School
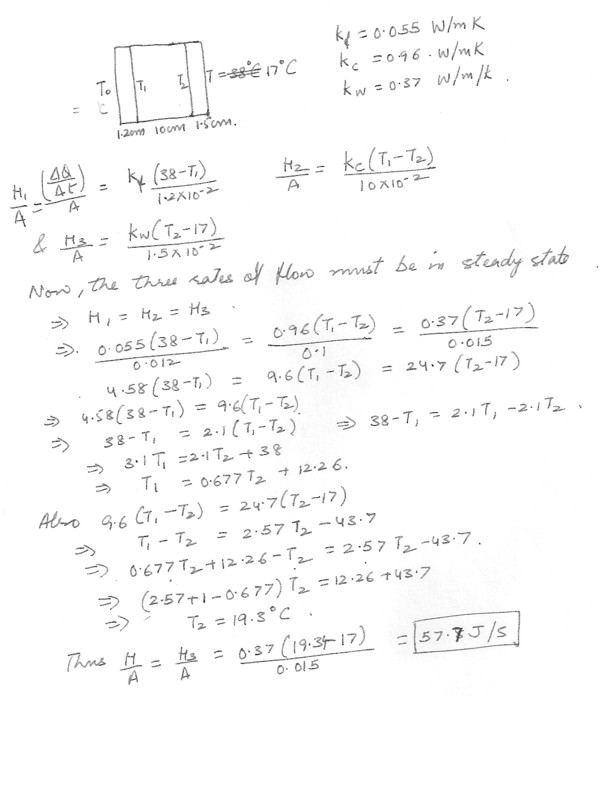
Conduction
Calculate the rate of heat transfer per unit are although a wall constructed of 12 mm fibro , 10.0 cm concrete and 15 mm of wood paneling if one side is at 17 degree C and the other is at 38 degree C .(K concrete = 0.96 , K fibro = 0.055 , K wood = 0.37 Wm-1K-1)
Physics Heat & Thermodynamics Level: High School
Thermal Expansion
In an winter (avg . temp 15 degree C) , Bob’s pendulum clock keeps time perfectly , where the pendulum has a period of 2 s , The pendulum shaft is a thin brass rod , which has a thermal expansion co-efficient of 2.0 x 10-5 K-1 , In one day , how much time will be lost /gained in the summer when the average temperature is 25 degree C ?The period of oscillation (T) for a pendulum of length l is given be ,
T = 2pi (sqrt g/g)
Where g is the acceleration due to gravity (g = 9.81 m/s2)

Physics Heat & Thermodynamics Level: High School
Specific Heat Capacity
Consider two equal mass samples made out of different material. Sample 1 has twice the specific heat capacity of sample 2 . To raise the temperature of the sample 1 from 50 degree C to 72 degree C requires 22 kJ. How many kJ are required to heat sample 2 from 90 degree C to 156 degree C .a) 33 kJ
b) 88 kJ
c) 132 kJ
d) 22 kJ
e) 66 kJ

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
How much taller is the Tower on a hot summer day ( 113 degree F) than on a cold (32 degree F) winter night ?
Physics Heat & Thermodynamics Level: High School
Choose all correct statements .a) A copper plate has a hole cut in its corner . When the plate is heated , the hole gets smaller .
b) When the pendulum of a grandfather clock is heated, the clock runs more slowly.
c) When the Celsius temperature doubles , the Kelvin temperature doubles .
d) When heat is added to a system , the temperature must rise .

Physics Heat & Thermodynamics Level: High School
The temperature of an ideal monatomic gas increases from 2 degree C to 4 degree C while remaining at constant pressure . Which of the following statements are true about the gas ?a) Heat must be taken from the gas
b) The gas does not work
c) The internal energy does not change
d) The volume decreases slightly
e) On a PV diagram , this process would be a horizontal line .

Physics Heat & Thermodynamics Level: High School
The chemical reaction below shows the conversion of 3 moles of ethyne to 1 mole of benzene .a) If the standard free energies of formation for ethyne and benzenerare 209 kJ mol-1 and 130 kJ mol-1 , respectively, determine by calculation whether this reaction is theoretically possible under standard conditions .
b) If it were possible for this reaction to occur , would the change in enthalpy (delta H) be greater than ,less than or the same as the change in internal energy (delta U) ?
c) Would the change in entropy for the reaction be positive ,negative or zero ?

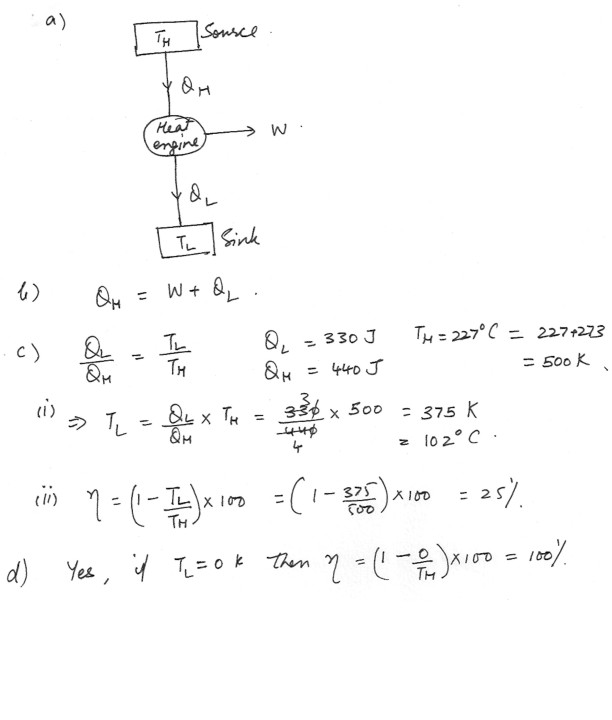
Physics Heat & Thermodynamics Level: High School
Heat Engine
a) Redraw the diagram which is shown at “click here” for the heat engine , and label the direction in which QH , QL and W are transferred .
b)What relationship would exist between QH, QL and W ?
c) While this engine operates on a Carnote cycle , it absorbs 440 of heat from the high temperature reservoir at 227 degree C , and it loses 330 J of heat to the low temperature reservoir .
(i) What is the value o TL ?
(ii) Calculate the efficiency of the engine .
d) If it were possible to have the low temperature reservoir at absolute zero , would the efficiency of the engine be 100 % ?
Physics Heat & Thermodynamics Level: High School
The cosmic background radiation is that of a black body at 2.7 K . What is the value lamda max at which the distribution has a maximum ? Answer in units of mm .

Physics Heat & Thermodynamics Level: High School
Heat Engine
An engine manufacturer makes the following claim : The heat input per second of the engine is 9.0kJ at 375K . The heat output per second is 4.0kJ at 225K . Do you believe these claims ? Explain why .
Physics Heat & Thermodynamics Level: High School
Determine the mean free path for nitrogen molecules with a diameter of 2.00*10^-10m at 20 degrees C and 1 atm .
Physics Heat & Thermodynamics Level: High School
Adiabatic Compression
A bicycle pump consists of cylinder 30cm long when the pump handle is all the way out . The pump contains air ( ã = 1.4) at 20 degrees C . If the pump outlet is blocked and the handle pushed until the internal length of the cylinder is 20 cm , by how much does the air temperature rise ? assume no heat loss .
Physics Heat & Thermodynamics Level: High School
An ideal gas is slowly compressed at a constant pressure of 2.0 atm from 10L to 20L . Then heat is added to the gas while holding the volume constant until the temperature reaches its original value . Calculatea) The total work done by the gas .
b) The total heat flow into the gas .

Physics Heat & Thermodynamics Level: High School
A star with the surface temperature of T = 10,000 K is going to be detected as more ____ than a star with T = 5,000 K located at the same distance from us .a) reddish and less luminous
b) reddish and more
c) bluish and more luminous
d) bluish and less luminous
e) none of the above

Physics Heat & Thermodynamics Level: High School
Principle of Calorimetry
What would the final temperature of 100 g of 20 degrees C water when 100 g of 40 degree C iron nails are submerged in it ?