Physics Heat & Thermodynamics Level: High School
Heat Engine
A heat engine does 7280 J of work in each cycle while absorbing 12.5 kcal of heat from a high-temperature reservoir . What is the efficiency (in percent) of this engine ?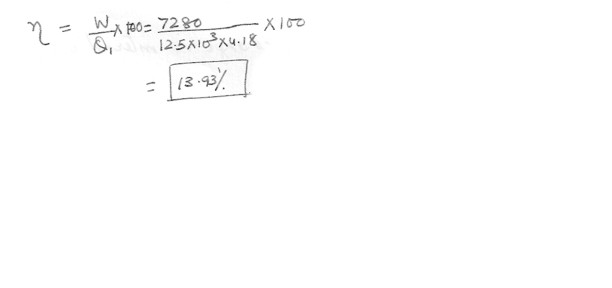
Physics Heat & Thermodynamics Level: High School
First Law of Thermodynamics
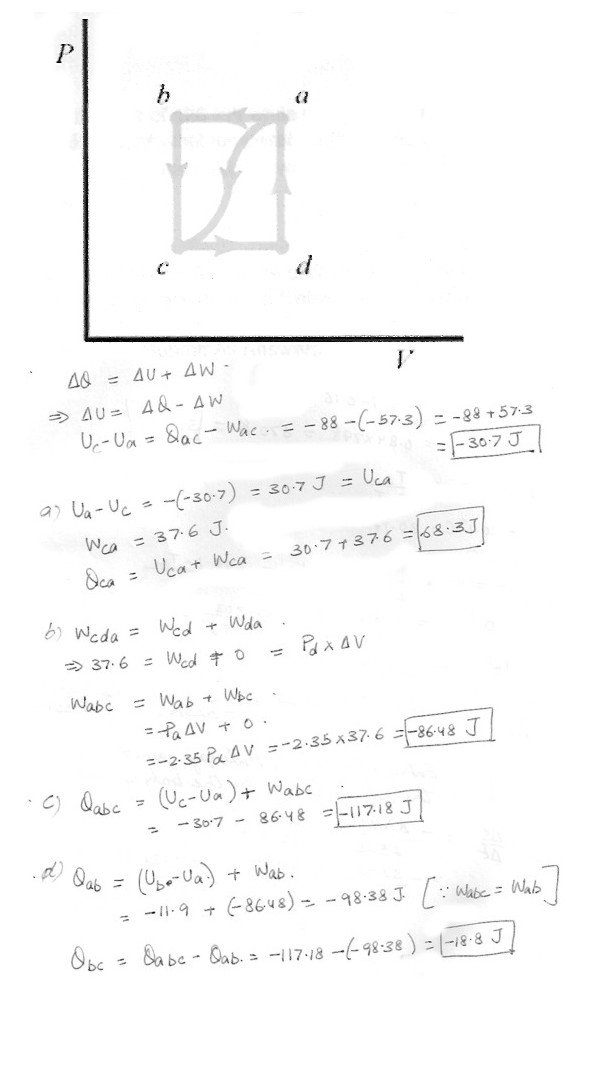
In the process of taking a gas from state a to state c along the curved path shown in the figure at ‘click here’, 88.0 J of heat leave the system and 57.3 J or work are done on the system .Determine the change in the internal energy , Uc-Ua . Here is a summery of what is given .
Qac = -88.0 J
Wac = -57.3 J
Wcda = 37.6 J
Ua-Ub = 11.9 J
Pa = 2.35 Pd
a) When the gas is taken along the path cda , the work done by the gas is W = 37.6 J . How much heat Q is added to the gas in the process cda ?
b) If Pa = 2.35 Pd, how much work is done by the gas in the process abc ?
c) What is Q for path abc ?
d) If Ua-Ub = 11.9 J , what is Q for the process bc ?
Physics Heat & Thermodynamics Level: High School
W.D in Isothermal process
An ideal gas expands isothermally, performing 5.26 x 10^3 J of work in the process . Calculate the change in internal energy of the gas .a) Calculate the heat absorbed during this expansion .

Physics Heat & Thermodynamics Level: High School
A match is placed in a oxygen-filled cylinder that has a movable piston . The piston is moved so quickly that no heat escapes as the match ignites . What kind of change is demonstrated in this process ?a) an isobaric change .
b) an adiabatic change .
c) an isothermal change .
d) an isochoric change .
e) a change of heat capacity .
Physics Heat & Thermodynamics Level: High School
Thermometry
Which one of the following properties could not be used as a temperature sensitive property in the construction of a thermometer ?a) The change in mass of a solid .
b) The change in volume of a liquid .
c) The change in length of a metal rod .
d) The change in electrical resistance of a wire .
e) The change in pressure of a gas at constant volume .
Physics Heat & Thermodynamics Level: High School
A match is placed in an oxygen-filled cylinder that has a movable piston . The piston is moved so quickly that no heat escapes as the match ignites . What kind of change is demonstrated in this process ?a) an isobaric change
b) an adiabatic change
c) an isothermal change
d) an isochoric change
e )a change of heat capacity
Physics Heat & Thermodynamics Level: High School
Change of state
Complete the following statement : when a substance undergoes fusion it .a) freezes
b) sublimes
c) condenses
d) vaporizes
e) evaporates
Physics Heat & Thermodynamics Level: High School
Thermal Expansion
A copper rod of diameter 2.0034 cm is cooled so that it fits into a circular hole of exactly 2.000 cm . How much doe the rod need to be cooled (in Celsius ) ?
Physics Heat & Thermodynamics Level: High School
Which of the following statement are true ?a) The Clausius form of the Second Law of Thermodynamics effectively says that heat will always flow from a colder to a hotter environment .
b) The Third Law of Thermodynamics effectively says that one can never reach a temperature of 0 K
c) The concept of temperature is introduced by the Zeroth Law of Thermodynamics.
d) The three mechanism of energy transfer by heat are convolution , convocation , and radiation .
e) One form of the Second Law of Thermodynamics is that all naturally occurring processes , the entropy of the universe always increases .

Physics Heat & Thermodynamics Level: High School
Calorimetry
To help keep the barn warm on winter days , a farmer stores 750 kg of warm water (20 degree C) in a large vat in the barn . How many hours would a 2.0 kW electric beater have to operate in order to provide the same amount of heat as is given off by the water as it cools down from 20.0 degree C to 0 degreeC and then completely freezes at 0 degree C. (Information about ice and water : c(water) = 4186 J/kg-K , c(ice)= 2090 J/kg-K, Lf(water) = 3.35 x 10^5 J/kg.)a) 30.2
b) 34.2
c) 38.6
d) 43.6
e) 49.3

Physics Heat & Thermodynamics Level: High School
Calorimetry
In the above problem, imagine that copper pellets (with the same mass per pellet)were used instead of lead pellets, In this case , to achieve the same equilibrium temperature , how many copper pellets would you need , compared to the original number of lead pellets ? Which of the following statements are true ?a) You need fewer copper pellets, but more than half the original number
b) You need more copper pellets, in fact, more than double the original number
c) You need fewer copper pellets , less than half the original number
d) You need the same number of copper pellets as silver originally
e) You need more copper pellets, but less than double the original number

Physics Heat & Thermodynamics Level: High School
Calorimetry
Small 1.1 g pellets of lead are heated to a temperature of 90 degree C and then added to a water bath containing 223 grams of water at 23 degree C . How many lead pellets must be added to the water in order to make the equilibrium temperature of the system 30 degree C ? (Specific heats of some metals in J/kg-K: lead (128), silver (234), copper (387) , iron(448) , aluminum (900))a) 582
b) 773-
c) 1029
d) 1368
e) 1820

Physics Heat & Thermodynamics Level: High School
Calorimetry
The specific heat of alcohol is about half that of water . Suppose that you have 0.5 kg of alcohol at room temperature (20 degree C) and 0.5 kg of hot water at 80 degreeC in separate containers . If you now mix these two liquids and allow them to come to thermal equilibrium, what will be the approximate equilibrium temperature (in degree C) of the mixture ? Which of the following statements are true ?a) 60 deg
b) 40 deg
c) 80 deg
d) 50 deg
e) 30 deg

Physics Heat & Thermodynamics Level: High School
Change of Phase
A 21.50 kg block of ice at alcohol of ice at -3.2 degree C slides on a horizontal surface with a coefficient of kinetic friction equal to 0.25 . The initial speed of the block is 6.90 m/s and its final speed is 2.30 m/s . Assuming that all the energy dissipated by kinetic friction goes into melting part of the ice , determine the mass of ice (in grams) that melts , (Information about ice and water : c (water) = 4186 J /kg-K , c(ice) = 2090 J/kg-K, Lf(water) = 3.35 x 10^5 J/kg) .a) 0.85
b) 1.07
c) 1.33
d) 1.66
e) 2.08

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
The pendulum in a grandfather clock is made of brass and keeps perfect time at 18.6 degrees C . How much time (in minutes) is gained or lost in a year if the clock is kept at 25.9 degree C ?(you may consider the clock to be a simple pendulum) (coefficient of linear expansion for brass is 1.9 x 10^-5)a) -19.5
b) -22.8
c) -26.7
d) -31.2
e) -36.5

