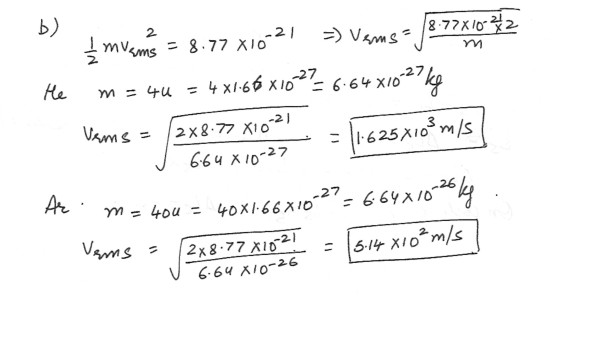Physics Heat & Thermodynamics Level: High School
A steam engine operates between 550 degrees and 300 degrees C . What is the maximum possible efficiency of this engine ?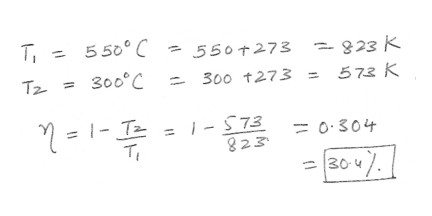
Physics Heat & Thermodynamics Level: High School
A steam engine operates between 550 degrees and 300 degrees C . What is the maximum possible efficiency of this engine ?
Physics Heat & Thermodynamics Level: High School
Work done in an adiatatic process
In an engine , 0.30 moles of an ideal monatomic gas in the cylinder expands rapidly and adiabatically against the piston . In the process , the temperature of gas drops from 1100 K to 45 K . How much work does the gas contain ?
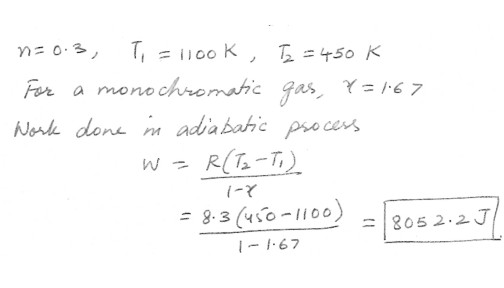
Physics Heat & Thermodynamics Level: Middle School
One and one half moles of an ideal monatomic gas expand adiabatically, performing 7500 J of work in the process. What is the change in temperature of the gas during this expansion?
Physics Heat & Thermodynamics Level: High School
A water heater can generate 32,000 kJ/h. How much water can it heat from 15 degrees Celsius to 50 degrees Celsius per hour?
Physics Heat & Thermodynamics Level: High School
The density of water at 4 degrees Celsius is 1.00 x 10^3 kg/m^3. what is water's density at 94 degrees Celsius?
Physics Heat & Thermodynamics Level: High School
A person eats a container of yogurt . The Nutritional Facts label states that it contains 195 Calories ( 1 calorie = 4186 J)What mass of perspiration would one have to lose to get rid of this energy ? At body temperature ,the latent heat of vaporization of water is 2.42 x 10^6 J/kg .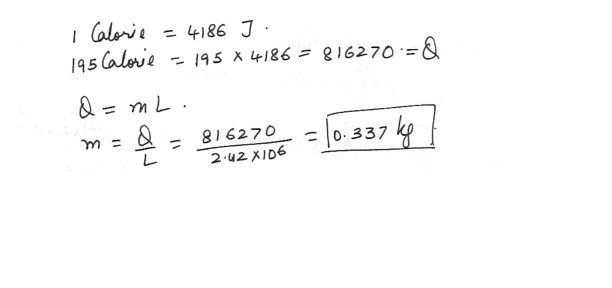
Physics Heat & Thermodynamics Level: High School
Ideal Gas Equation
a) An ideal gas occupies a volume of 2.8 cm^3 at 20 degree C and atmospheric pressure . Determine the number of molecules of gas in the container .
b) If the pressure is reduced to 2.6 x 10^-11 Pa ( an extremely good vacuum) while the temperature remains constant , how many moles of gas remain in the container ?

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
A hollow aluminum cylinder 23.0 cm deep has an internal capacity of 2.00 L at 20.0 degree C . It is completely filled with turpentine and then warmed to 74.0 degree C .a) How much turpentine overflows ?
b) If it is then cooled back to 20.0 degree C , how far below the surface of the cylinder’s rim is the turpentine’s surface ?

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
On a day when the temperature is 20.5 degree C , a concrete walk is poured in such a way that its ends are unable to move .a) What is the stress in the cement when its temperature is 49.5 degree C ?

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
On a day when the temperature is 20.5 degree C , a concrete walk is poured in such a way that its ends are unable to move .a) What is the stress in the cement when its temperature is 49.5 degree C ?

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
The average coefficient of volume expansion for carbon tetrachloride is 5.81 x 10^-4 (degree C)^-1 . If a 49.5 gal steel container is filled completely with carbon tetrachloride when the temperature is 10.0 degree C , how much will spill over when the temperature rises to 30.0 degree C ?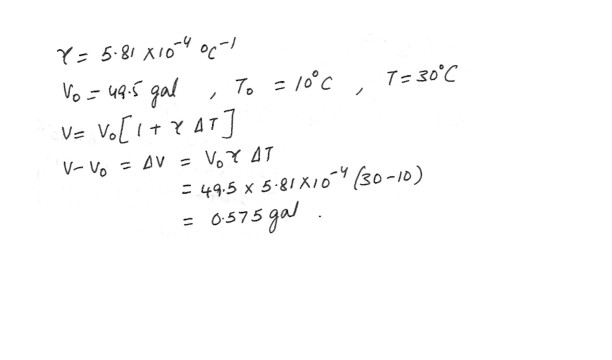
Physics Heat & Thermodynamics Level: High School
Thermal Expansion
A steel measuring tape was designed to read correctly at 20 degree C . A parent uses the tape to measure the height of a 1.2 m tall child . If the measurement is made on a day when the temperature is 28 degreeC , is the tape reading longer or shorter than the actual height , and by how much ?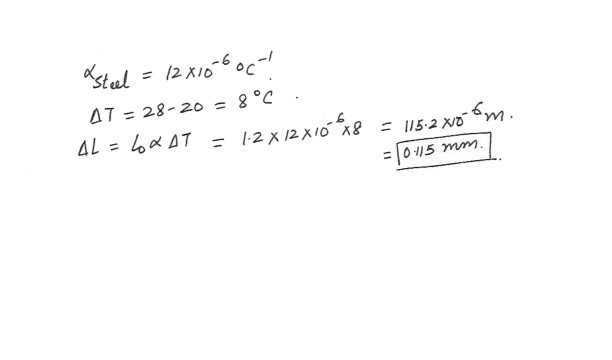
Physics Heat & Thermodynamics Level: High School
Thermal Expansion
A copper rod and steel rod are heated . At 0 degree C the copper rod has a length o Lc, the steel one has a length Ls . When the rods are being heated or cooled , a difference of 3.50 cm is maintained between their lengths . Determine the values of Lc and Ls .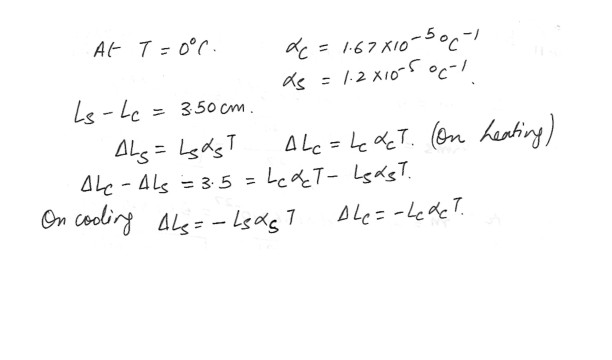
Physics Heat & Thermodynamics Level: High School
Average Kinetic Energy of a molecule
A cylinder contains a mixture of helium and argon gas in equilibrium at a temperature of 151 degree C .a) What is the average kinetic energy of each type of molecule ?
b) What is the rms speed of each type of molecule ?