Physics Heat & Thermodynamics Level: University
A heat pump is used to heat a building. The outside temperature is - 5 C and the temperature inside the building is to be maintained at 22 C. The pump's coefficient of performance is 3.8 and the the heat pump delievers 7.54 MJ of heat to the building each hour. If the heat pump is a Carnot engine working in reverse at what rate must work be done to run the heat pump?

Physics Heat & Thermodynamics Level: University
Efficiency of engineA inventor claims to have constructed an engine that has an efficiency of 75 percent when operated between the boiling and freezing points of water. Is this possible? Explain.

Physics Heat & Thermodynamics Level: University
Specific Heat & Latent HeatFind the minimum amount of energy required to completely melt at 130 g of silver initially at 15.0 C.

Physics Heat & Thermodynamics Level: University
A steel rod is 3.000 cm in diameter at 25 C. A brass ring has an interior diameter of 2.992 cm at 25 C. At what common temperature will the ring just slide onto the rod?

Physics Heat & Thermodynamics Level: University
EntropyA 2.0 kg block of lead melts at its melting point of 327.3 C. Calculate the change in its entrophy.
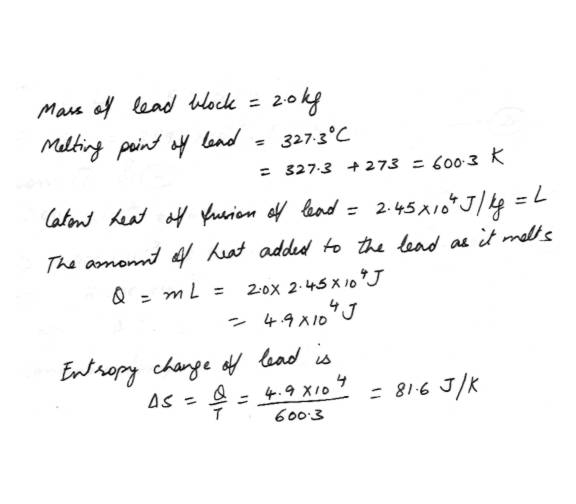
Physics Heat & Thermodynamics Level: University
HeatA Carnot engine takes in 100 J at 100 degrees C. At the end of the cycle 50 J are exhausted. What is the temperature of the exhaust.

Physics Heat & Thermodynamics Level: University
HeatA Carnot engine operating at 30 percent efficiency exhausts heat into a heat sink at 283 C. By how much would the temperature of the heat reservoir need to be raised to increase the engine efficiency to 45 percent .

Physics Heat & Thermodynamics Level: University
HeatIf 120 g of hot coffee at 85 C is poured into an insulated cup containing 200 g of ice (a) How many grams of liquid will there be when the system reaches thermal equilibrium.
(b) How much ice will remain

Physics Heat & Thermodynamics Level: University
If the energy that goes onto evaporating 1kg of water from a lake were used instead to raise that same kg into the air how high would the water be raised?
Physics Heat & Thermodynamics Level: University
HeatAn iron bar is exactly 1 meter long at 15 degree celcius. A brass bar is 0.5 mm shorter at the same temperature. At what temperature will they be the same lengths if they are heated simultaneously.

Physics Heat & Thermodynamics Level: University
HeatAn iron bar is exactly 1 meter long at 15 degree celcius. A brass bar is 0.5 mm shorter at the same temperature. At what temperature will they be the same lengths if they are heated simultaneously.

Physics Heat & Thermodynamics Level: University
HeatA steel plate with a circular hole 2.01 cm in radius and a copper ball with a radius of 2 cm are initially at 15 degree celcius . If their temperature is raised to 275 degree celcius , will the ball still fit through the hole?

Physics Heat & Thermodynamics Level: University
Thermal expansionZirconium tungstate is an unusual material as its volume shrinks with an increase in temperature for the temperature range 0.3 k to 1050 K ( where it decomposes). The volumetric coefficient of thermal expansion is -26.4 x 10 -6 / K. What is the ratio Delta V upon V not for the above temperature range ( in percentage)?

Physics Heat & Thermodynamics Level: Middle School
A metal rod 40.0000 cm long at 40 degree C is heated to 60 degree C. The length of the rod is then measured to be 40.0105 cm. What is the coefficient of linear expansion of the metal in units of / C?
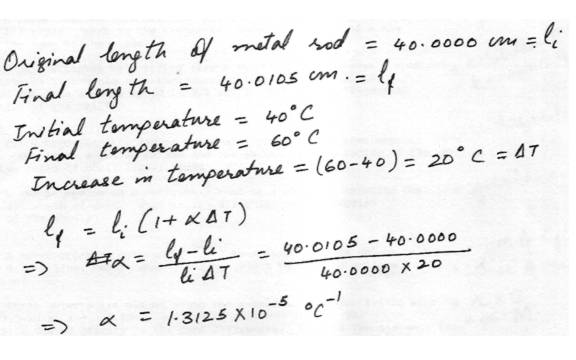
Physics Heat & Thermodynamics Level: University
GasUsing the value of atmospheric pressure at sea level 1.0 x 105 pa estimate the total mass of the earths atmosphere above a area.

