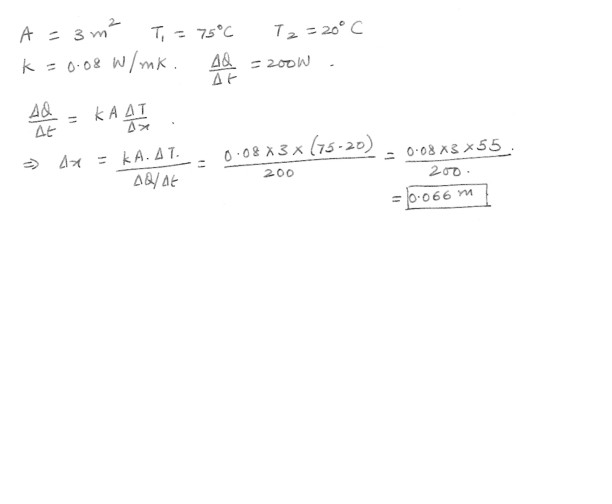Physics Heat & Thermodynamics Level: High School
If n moles of an ideal gas are compressed isothermally from an initial volume V1 to a final volume V2 , the change in entropy isa) nR In (V2/V1)
b) nRT In (V2/V1
c) nk In (V2/V1)
d) nCv/T
Physics Heat & Thermodynamics Level: High School
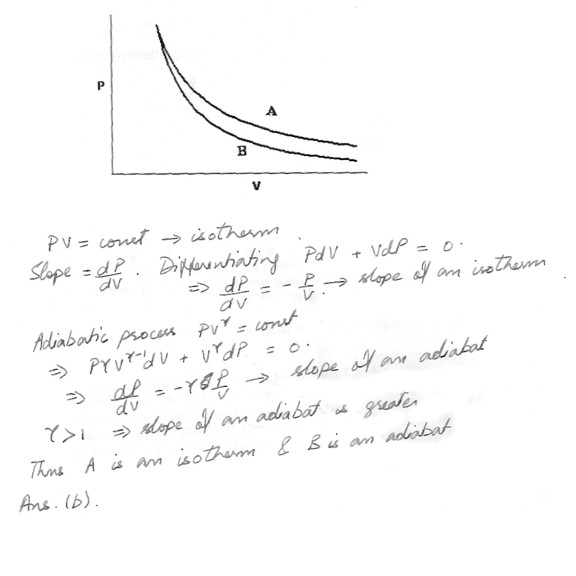
The relation PV = nRT holds for all ideal gases . The additional relation (PV = constant ) holds for an adiabatic process . The figure below shows two curves : one is an adiabat and the other is an isotherm . Each starts at the same pressure and volume . Which statement is correct ( Note : ‘tidel’ means is proportional to.)a) Isotherm : P tidel 1/V ; Adiabet : P tidel 1/V; B is both isotherm and an adiabet .
b) Isotherm : P tidel 1/V ; Adiabat : P tidel 1/V ; A is an isotherm , B is an adiabat .
c) Isotherm : P tidel 1/V ; Adiabat : P tidel 1/V ; B is an isotherm , A is an adiabat .
d) Isotherm : P tidel 1/V; Adiabat : P tidel 1/V; A is both an isotherm and an adiatbat .
e) Cannot answer this without additional information about the starting temperature .
Physics Heat & Thermodynamics Level: High School
A molecule in a uniform ideal gas can collide with other molecules when their center are equal to or less than :a) One radius away from center .
b) One diameter away from center .
c) Two diameter away from its center .
d) Twice the cube root of volume away from its center .
e) Sqrt 3 diameters away from its center .
Physics Heat & Thermodynamics Level: High School
Assume 3 moles of a diatomic gas has an internal energy of 10 kJ . Determine the temperature of the gas after it has reached equilibrium .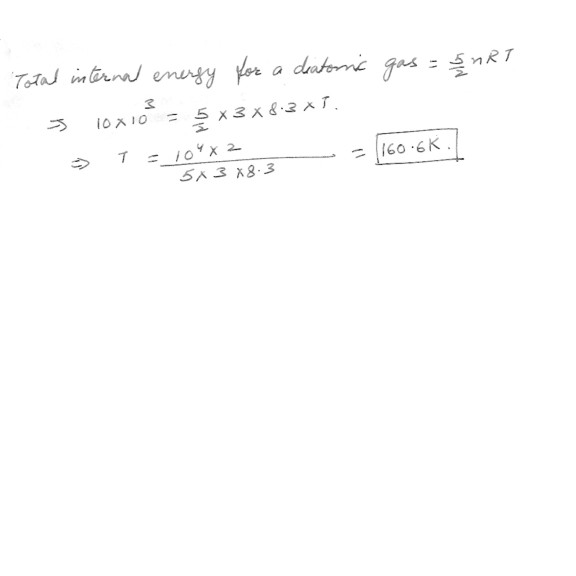
Physics Heat & Thermodynamics Level: High School
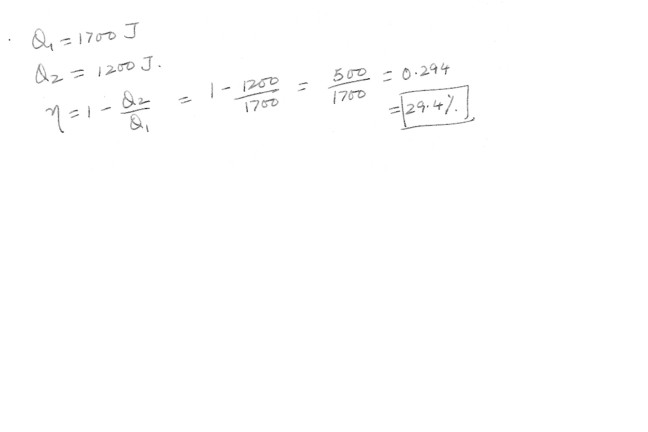
An engine absorbs 1700 J from a hot reservoir and expels 1200 J to a cold reservoir in each cycle .a) What is the engine’s efficiency ?
b) How much work is done in each cycle ?
c) What is the power output of the engine if each cycle lasts for 0.300 s ?
Physics Heat & Thermodynamics Level: High School
A heat engine operates between two reservoirs at temperatures of 20 degrees C and 300 degrees C . What is the maximum efficiency possible for this engine ?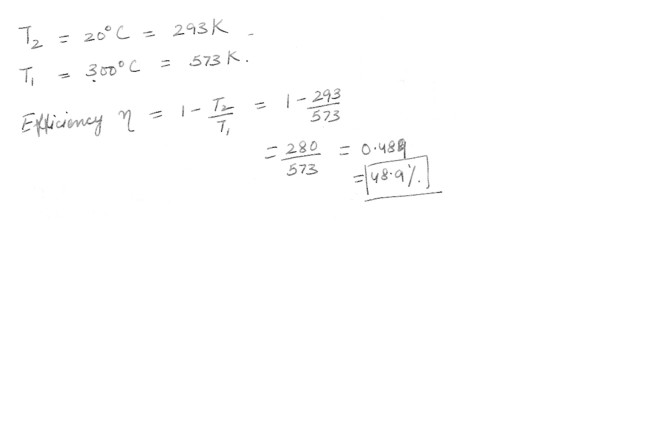
Physics Heat & Thermodynamics Level: High School
A 5.0 kg block of aluminum is heated from 20 degrees C to 90 degrees C a atmospheric pressure . Finda) The work done by the aluminum
b) The amount of energy transferred to it by heat
c) The increase in its internal energy

Physics Heat & Thermodynamics Level: High School
Sketch a PV diagram of the following processes :a) A gas expands at constant pressure P1 from volume V1 to volume V2 . It is then kept at constant volume while the pressure is reduced to P2 .
b) A gas is reduced in pressure from P1 to P2 while its volume is held constant at V1 . It is then expanded at constant pressure P2 to a final volume V2 .
c) In which of the processes is more work done by the gas ? why ?
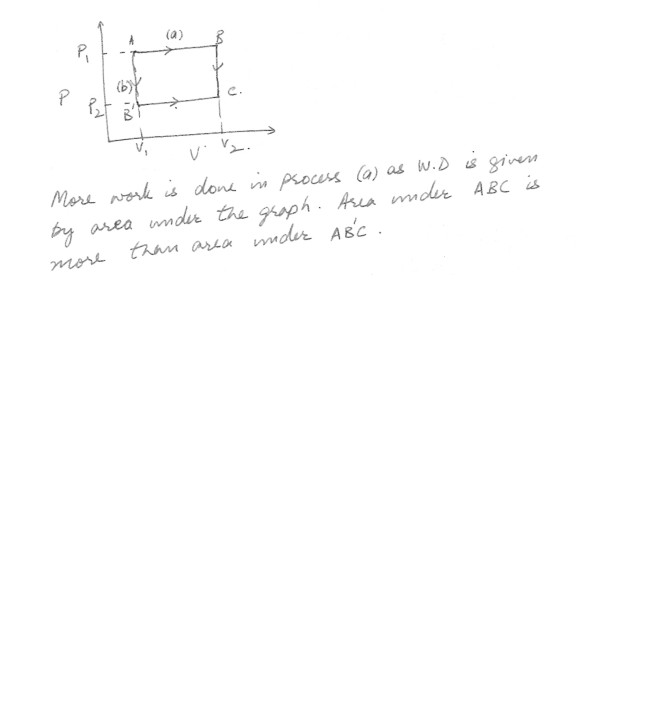
Physics Heat & Thermodynamics Level: High School
One mole of a monatomic idea gas at and initial pressure of 4 atm and temperature of 300 K is carried though the following reversible cycle :1) It expands isothermally until it volume is doubled ;
2) It is compressed to its original volume at constant pressure ;
3) It is compressed isothermally to a pressure of 4 atm; and
4) It expands at constant pressure to its original volume .
a) Make an accurate plot of the cyclic process on a PV diagram .
b) Calculate the work done by the gas per cycle .
c) What is the efficiency of an engine operating in this cycle ?
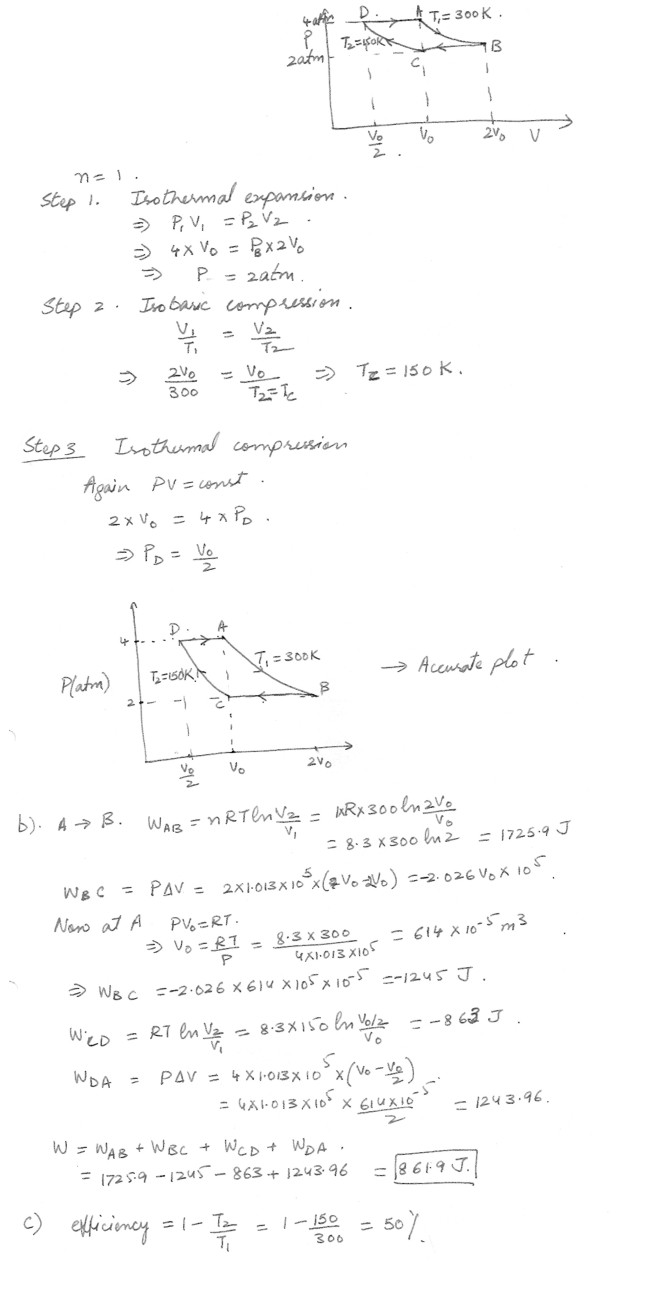
Physics Heat & Thermodynamics Level: High School
The figure at “click here” represents n moles of an ideal monatomic gas being taken through a reversible cycle consisting of two isothermal processes at temperature 3To and To and two constant-volume processes . Determine in terms of n, R, and To (a) the net heat transferred to the gas and (b) the efficiency of an engine operating in this cycle .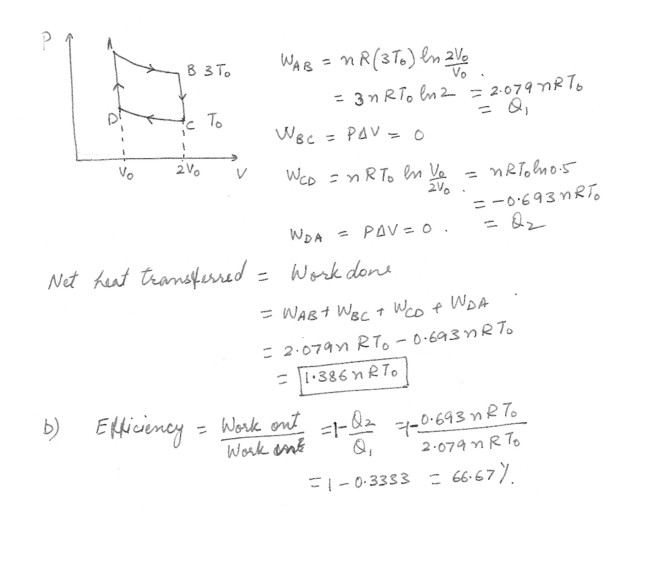
Physics Heat & Thermodynamics Level: High School
An aluminum rod conducts 8.40 cal/s from a heat sources maintained at 2.10E 2 degrees C to a large body of water at 24 degrees C . Calculate the rate entropy increases per unit time in this process .
Physics Heat & Thermodynamics Level: High School
A heat engine utilizes a heat source at 5.4E 2 degrees C and has an ideal (Carnot) efficiency of 25% . To increase the ideal efficiency to 36% ,what must be the temperature of the heat source ?
Physics Heat & Thermodynamics Level: High School
Air modeled as an ideal diatomic gas (y = 1.40) goes through the following cycle :1„³ 2 Constant pressure expansion from P1 = 1000 kPa , V1 = 0.200 m^3 to V2 = 0.400 m^3. T1 = 800 K .
2„³3 Adiabatic expansion to V3 = 0.800 m^3 .
3„³4 Constant pressure compression
4„³1 Adiabatic compression to the original state .
1) Find the work and heat transfer for each process . Determine whether the work or heat transfer is positive or negative .
2)Determine the total work done in the cycle .
3)Determine the total heat transfer in the cycle .
4)Sketch the process on a PV diagram .

Physics Heat & Thermodynamics Level: High School
1 kg of ice at -30 degrees C is converted to steam at 120 degrees C . Calculate the heat transfer for each process . Determine the total heat transfer . Calculate the entropy change for each process . Determine the total entropy change . .
Physics Heat & Thermodynamics Level: High School
Conduction
A water heater is covered with insulation boards over a total surface area of 3.00 m^2 . The inside board surface is at 75 degrees C , the outside surface is at 20 degrees C , and the board material has a conductivity of 0.08 W/m.K . How thick should the board be to limit the heat transfer loss to 200 W ?