Physics Heat & Thermodynamics Level: High School
An engine goes through the following cycle with 3.00 moles sample of a diatomic gas .(i) An isothermas expansion from point a to point b
(ii) a constant pressure compression from point b to point c .(iii) an adiabatic compression from point c to point a . we giving point a has a temperature 498 K , and a volume 1.40 x 10^-3 m^3 .
a) What is the pressure in Pascals at point a ?
b) What is the pressure in Pascals at point b if the volume of point b is 0.270 m^3 ?
c) What is the volume at point c ?
d) What is the temperature at point c ?
e) What is the change in internal energy for process II (constant pressure ) ?
f) What is the work done in process II ?
g) What is the heat transfer in process II ?

Physics Heat & Thermodynamics Level: High School
Calorimetry
An insulated beaker with negligible mass contains liquid water with a mass of 0.220 kg and a temperature of 85.7 degrees C . How much ice at a temperature of -20.3 degrees C must be dropped into the water so that the final temperature of the system will be 23.7 degrees C ?Calculate the heat lost by the water when cooled to Tf . Determine an expression for the heat gained by the ice in terms of the mass of ice . Since there is no heat added to or removed from the system , the total heat change of the system must be zero . Set up the approximate equation and solve for the mass of ice .
Take the specific heat of liquid water to be 4190 J/kg.K , the specific heat of ice to be 2100 J/kg.K , and the heat of fusion for water to be 334 kJ/kg .

Physics Heat & Thermodynamics Level: High School
1) Find the linear equation that expresses temperature in degrees Fahrenheit as a function of temperature in degrees Celsius .
2) Find the linear equation that expresses temperature in degrees Celsius as a function of temperature in degrees Fahrenheit .
3) How do the graphs of these two functions differ ?
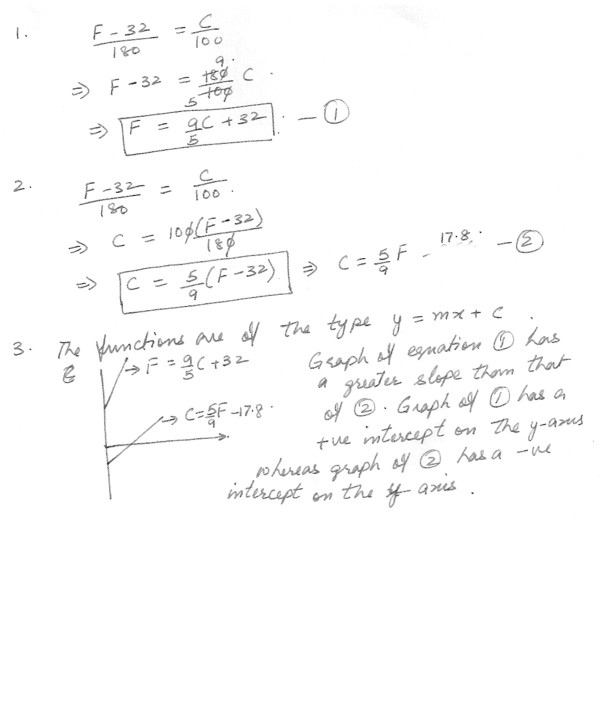
Physics Heat & Thermodynamics Level: High School
There is a formula that converts temperature in degrees Celsius to temperature in degrees Fahrenheit . You are given the following data points .Fahrenheit ---- Celsius
Freezing point of water 32---- 0
Boiling point of water 212 ---100

Physics Heat & Thermodynamics Level: High School
Conduction
a) Find the rate of energy flow through a copper block of cross sectional area 15 cm squared , and length 8.0 cm when a temperature difference of 30 degrees C is established across the block . Repeat the calculation , assuming that the material is b) a block of stagnant air with the given dimensions , c) a block of wood with the given dimensions .
Physics Heat & Thermodynamics Level: High School
Calorimetry
What mass of steam that is initially at 120 degrees C , is needed ot warm 350 g of water and its 300 g aluminum container from 20 degrees C to 50 degrees C ?
Physics Heat & Thermodynamics Level: High School
Calorimetry
A 100 g of cube of ice at 0 degrees C , is dropped into 1.0 kg of water that was originally at 80 degrees C . What is the final temperature of the water after the ice has melted ?
Physics Heat & Thermodynamics Level: High School
Radiation
A blackbody at 0 degree C has a surface are of 35 cm squared . Determine the rate at which energy is emitted by the object .
Physics Heat & Thermodynamics Level: High School
A ideal gas undergoes a process which increases its temperature from 20 degree C to 89 degreeC while increasing its gauge pressure from 0.00 atm to 4.78 atm . If the volume of the gas at the end of the process is 317 cm cubed , find the initial volume .
Physics Heat & Thermodynamics Level: High School
Suppose that the heat energy is being supplied to a section of an ice covered road by the sun at the rate of 25.0 kW . How much ice at 0 degree C can be melted in one hour ?
Physics Heat & Thermodynamics Level: High School
Calorimetry
55.0 g of H2O at 10 degrees C are mixed with 75.0 g of H2O in a 137 g copper cup (c = .093 cal/gC) at 20 degrees C . Determine the final temperature of the system at equilibrium
Physics Heat & Thermodynamics Level: High School
Helium in a toy balloon does work on its surroundings as it expands with a constant pressure of 3.42 x 10^5 Pa in excess of atmospheric pressure . The balloon’s initial volume is 2.8 x 10^-4 m^3 , and its final volume is 2.58 x 10^-3 m^3 . Find the amount of work done by the gas in the balloon .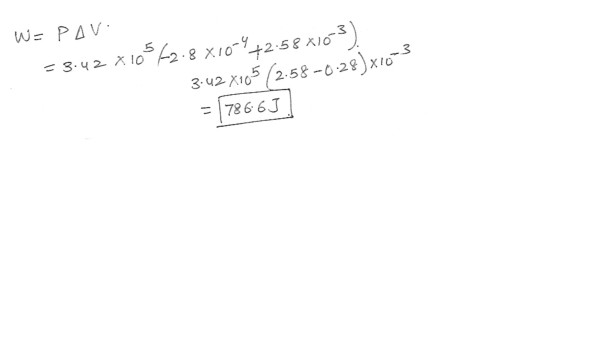
Physics Heat & Thermodynamics Level: High School
Given: Specific heat of water = 1 cal/(g x *C) : How much heat is required to vaporize a(n) 7g ice cube initially at 0*C ? The latent heat of fusion of ice is 80 cal/g and the latent heat of vaporization of water is 540k cal/g . Answer in units of cal .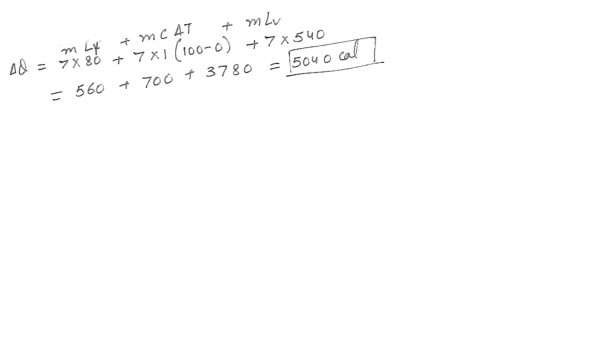
Physics Heat & Thermodynamics Level: High School
Given : Specific heat capacity = 4180 J = kg x *C and latent heat of vaporization of water = 2.26 x 10^6 J/kg . You have 99 g of steam at 100*C . How much heat must be removed to change it to 99 g of water at 21 *C .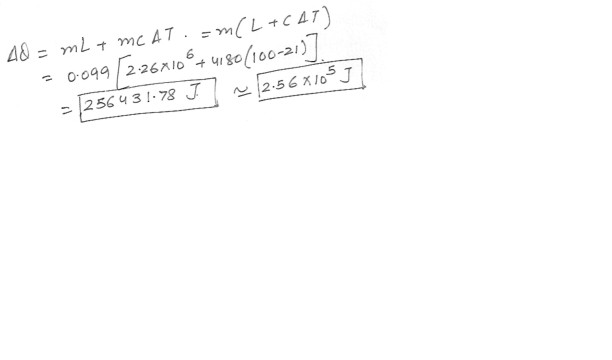
Physics Heat & Thermodynamics Level: High School
A 69.7 kg runner expends 457 J/s of power while running a marathon . Assuming that 10% of the energy is delivered to the muscle tissue and that the excess energy is primarily removed from the body by sweating , determine the volume of bodily liquid (assume it is water) lost per hour . (At 37*C the latent heat of vaporization of water is 2.41 x 10^6 J= kg) . Answer in units of cm^3 .
