Physics Heat & Thermodynamics Level: High School
You have collected exactly 2500 aluminum cans fro recycling ,each with a mass of 14.2 g . How much energy is needed to melt them if their initial temperature is 26.2*C respectively . Answer in units of J .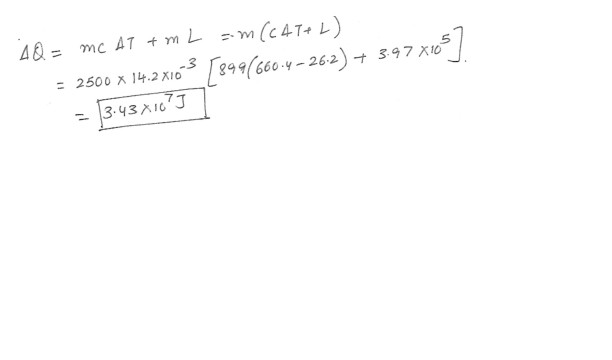
Physics Heat & Thermodynamics Level: High School
Conservation of Energy
A 777 kg car is moving at 27 m/s brakes to a stop . The brakes contain about 15 kg of iron that absorb the energy . What is increase in temperature of brakes ? Assume the specific heat of iron is 450 J/kg x degrees C . Answer in units of degrees C .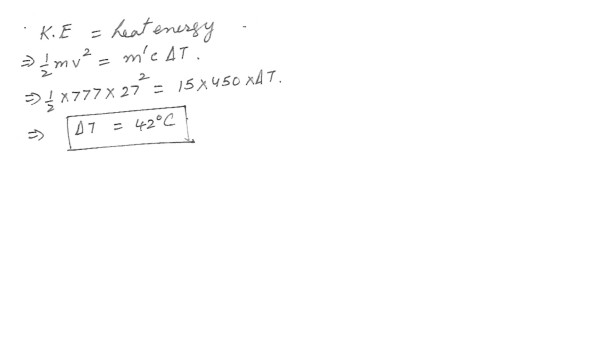
Physics Heat & Thermodynamics Level: High School
A 99.4 kg weight-watcher wishes to climb a mountain to work off the equivalent of a large piece of chocolate cake rated at 698 (food) Calories . (1 food Calorie = 10^3 calories) . The acceleration of gravity is 9.8 m/s^2 . How high must the person climb ? Answer in units of km . 23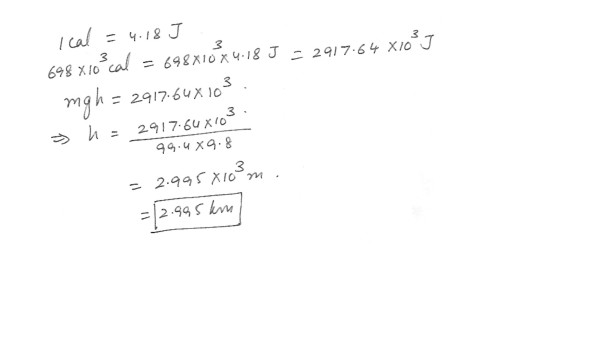
Physics Heat & Thermodynamics Level: High School
Calorimetry
Given : Specific heat of water = 4180 J/kg x degree C . You need to raise the temperature of 59 g water from 11.5 degrees C to 78 degrees C . How much heat is needed to accomplish this ? Answer in units of J .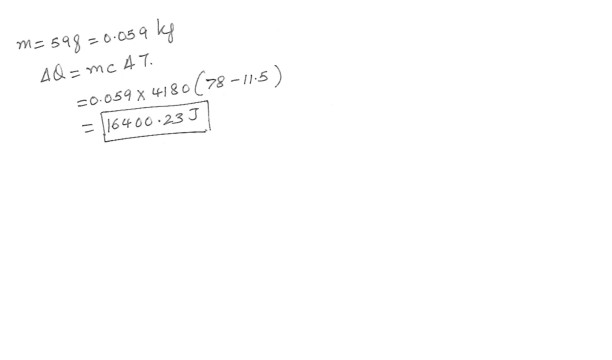
Physics Heat & Thermodynamics Level: High School
Calorimetry
What is the final equilibrium temperature when 17 g of milk at 19 degrees C is added to 101 g of coffee at 89 degrees C ? Assume the specific heats of milk and coffee are the same as that of water , and neglect the specific heat of the container , Answer in units of degrees C .
Physics Heat & Thermodynamics Level: High School
A helium-filled balloon has a volume of 0.5 m^3 . As it rises in the earth’s atmosphere , its volume changes , what is its new volume if its original temperature and pressure are 12degrees C and 1.6 atm and its final temperature and pressure are (-22degrees C) and 0.6 atm ? Answer in units of m^3 .
Physics Heat & Thermodynamics Level: High School
First law of Thermodynamics
An ideal gas is compressed from 20 liters to 2.0 liters at a constant pressure of 3.0 atm by rawing from it so that the temperature drops . At that point , its volume is held constant and heat is added to it so that the pressure rises and its returns to the original temperature . Draw a P.V diagram . Compute the network done on or by the gas determine the net heat flow .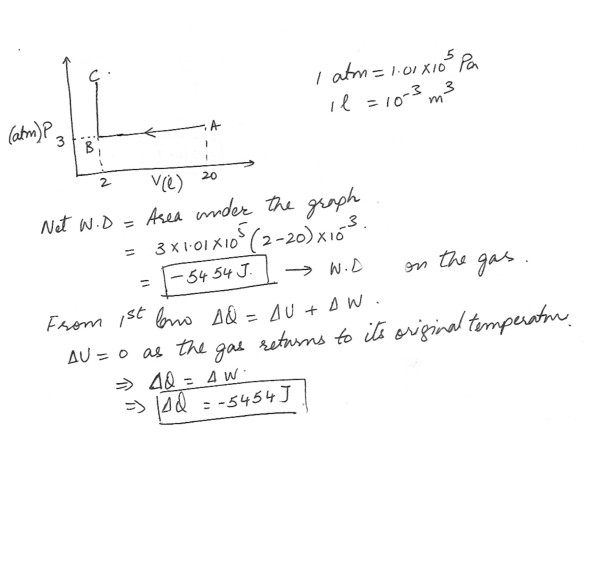
Physics Heat & Thermodynamics Level: High School
A 5.0 kg piece of aluminum at 350 degrees C is dropped on to 3 kg of ice . All the ice melts . What is the final temperature of the water ?
Physics Heat & Thermodynamics Level: High School
Calorimetry
Two grams of liquid water are at 0 degree C , and another five grams are at 100 degrees C . Heat is removed from the water at 0 degree C , completely freezing it at 0 degree C . This heat is then used to vaporize some of the water at 100 degrees C . What is the mass (in grams) of the liquid water that remains ?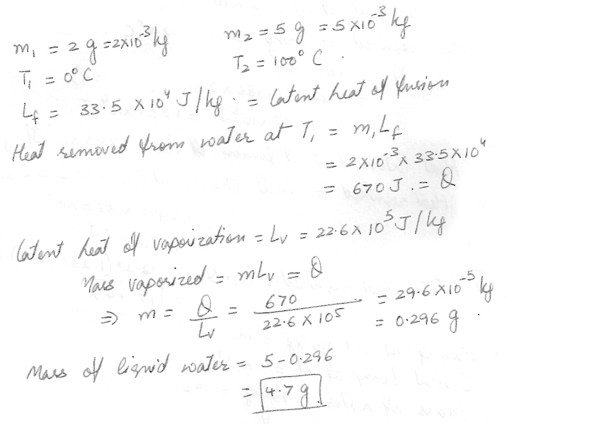
Physics Heat & Thermodynamics Level: High School
Calorimetry
A 0.215 kg piece of aluminum that has a temperature of -155 degrees C is added to 1.5 kg of water that has a temperature of 3.0 degrees C . At equilibrium the temperature is 0.0 degree C . Ignoring the container and assuming that the heat exchanged with the surroundings is negligible , determine the mass of water that has been frozen into ice .
Physics Heat & Thermodynamics Level: High School
A woman finds the front windshield of her car covered with ice at -12.4 degree C . The ice has a thickness of 4.35 x 10^-4 , and the windshield has an area of 1.25 m^2 . The density of ice is 917 kg/m^3 . How much heat is required to melt the ice ?
Physics Heat & Thermodynamics Level: High School
The latent heat of vaporization of H2O at body temperature (37 degreeC ) is 2.42 x 10^6 J/kg . To cool the body of a 72 kg jogger (average specific heat capacity = 3500 J/(kg . degree C) by 1.2 degree C , how many kilograms of water in the form of sweat have to be evaporated ?
Physics Heat & Thermodynamics Level: High School
Calorimetry
At a fabrication plant , hot metal forging has a mass of 79 kg and a specific heat capacity of 430 J/(kg.degrees C) . To harden it , the forging is immersed in 710 kg of oil that has a temperature of the oil and forging at thermal equilibrium is 46 degree C . Assuming that the heat flows only between the forging and the oil , determine the initial temperature of the forging .
Physics Heat & Thermodynamics Level: High School
Calorimetry
2 objects are added to a container holding 200 g of 20 deg C ethanol . The hot object has a mass of 100 g and is removed from a pot of 212 deg F water , its specific heat being 1/5 that of water . The other object is taken from a 263 K freezer , its mass is 75 g and its specific heat 30% that of water . What will be the final equilibrium temperature of the mixture ?
Physics Heat & Thermodynamics Level: High School
Surface area of a city is 45 square miles with a snow density of 15% that of water . Average depth of snow is 13 inches . What is the volume in cubic meters of snow covering the city ? How much energy in both joules and kcal would be needed to turn this snow (32 deg F ) into water ? Assume 9 hours of sunlight and average sunlight intensity is 400 W/meter squared . Also assume that 50% of this energy is absorbed by the snow . How many hours of sunlight is needed to melt the snow ?
