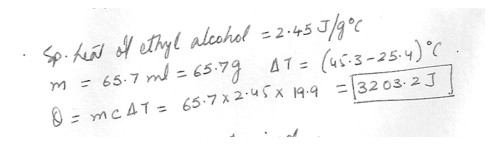Physics Heat & Thermodynamics Level: High School
An ideal gas is compressed to one-half its original volume during an isothermal process . The final pressure of the gas :a) increase to twice its original value .
b) increases to less than twice its original value .
c) increase to more than twice its original value .
d) does not change
e) increased to 4 times its original value .

Physics Heat & Thermodynamics Level: High School
An idea gas is compressed isothermally from 30 L to 20 L . During this process , 6.0 J of energy is expended by the external mechanism that compressed the gas . What is the change of internal energy for this gas ?a) 6.0 J
b) zero
c) -6.0 J
d) 36.0 J
e) none of the above
Zero
(in a isothermal process , there is no change in the internal energy )Physics Heat & Thermodynamics Level: High School
Consider two neighboring rectangular houses built from the same materials . One of the house has twice the length , width, and height of the other . Under identical climate conditions , what would be true about the rate that heat would have to be supplied to maintain the same inside temperature on a cold day ? Compared to the small house , the larger house would need heat supplies at :a) twice the rate
b) 4 times the rate
c) 8 times the rate
d) 16 times the rate
e) none of the above

Physics Heat & Thermodynamics Level: High School
Convection can occur :a) only in solids
b) only in liquids
c) only in gases
d) in solids , liquids, and gases
e) only in liquids and gases
Physics Heat & Thermodynamics Level: High School
On a cold day , a piece of metal feeds much colder to the touch than a piece of wood . This is due to the difference in which one of the following physical property ?a) density
b) specific heat
c) latent heat
d) thermal conductivity
e) specific gravity
Physics Heat & Thermodynamics Level: High School
If s well known fact that water has a higher specific heat capacity than iron . Now , consider equal masses of water and iron that are initially in thermal equilibrium . The same amount of heat , 30 calories , is added to each . Which statement is true.a) They remain in thermal equilibrium
b) They are no longer in thermal equilibrium ; the water is warmer .
c) They are longer in thermal equilibrium ; the iron is warmer .
d) It is impossible to say without knowing the exact mass involved and the exact specific heat capacities .

Physics Heat & Thermodynamics Level: High School
The reason that ocean temperature do not vary drastically is that :a) water has a relative high rate of heat conduction .
b) water is good radiator
c) water is relatively high specific heat .
d) water is poor heat conductor.
e) water is a poor convection media
Physics Heat & Thermodynamics Level: High School
A cup of water is scooped up from a swimming pool of water . Compare the temperature T and the internal energy U of the water in both the cup and the swimming pool .a) T (pool) is greater than T (cup) , and the U is the same .
b) T(pool) is less than T(cup) , and U is the same .
c) T(pool) is equal to T(cup) , and U(pool) is greater than U (cup)
d) T(pool) is equal to T(cup) , and U(pool) is less than U(cup)
e) Both (a) and (b) are correct .
Physics Heat & Thermodynamics Level: High School
A block of ice at 0 degree is added to a 150 g aluminum calorimeter cup that holds 200 g of water at 10 degree C . If all but 2.00 g of ice melt , what was the original mass of the block of ice ?
Physics Heat & Thermodynamics Level: High School
Determine the latent heat of vaporization of unknown substance X in kcal/g if 3.0 g of boiling liquid X are completely vaporized in 1.5 hours by an energy source of 10 k cal/h .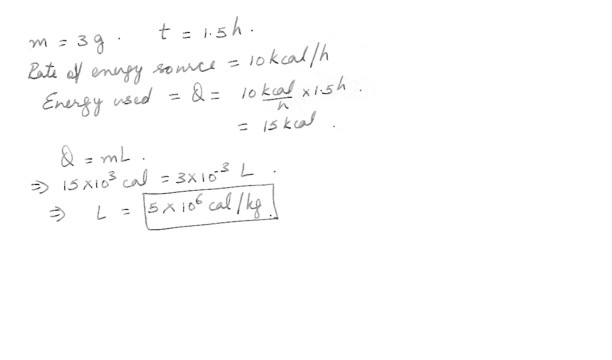
Physics Heat & Thermodynamics Level: High School
Two spheres , labeled A and B , have identical masses , but are made of different substances . The specific heat capacity of sphere A is 440 J / kg degree C and that of sphere B is 160 J/kg. degree C . These spheres are initially at 21 degree C ; and the same quantity of heat is added to each sphere . If the final temperature of sphere A is 72 degree C , what is the final temperature of sphere B ?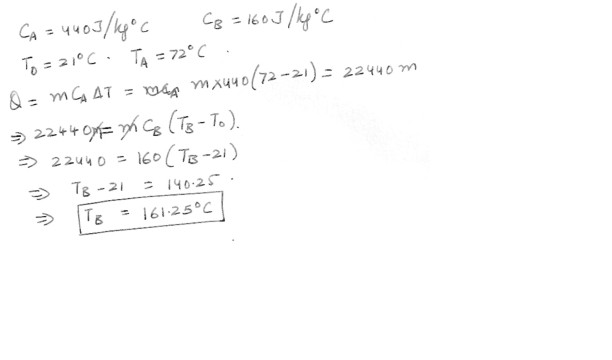
Physics Heat & Thermodynamics Level: High School
A soft drink manufacturer claims that the new diet drink is “low Joule” . The label indicates the available energy is 6300 J . What is the equivalent of this energy in Calories (1 Calorie = 1000 Cal ) ?
Physics Heat & Thermodynamics Level: High School
Two cubes , one silver and one iron , have the same mass and temperature . A quantity Q of heat is removed from each cube . Which one of the following properties cause the final temperature of the cubes to be different ?a) Density
b) Latent heat vaporization
c) Specific heat capacity
d) Coefficient of volume expansion
e) Volume
Physics Heat & Thermodynamics Level: High School
If 45.3 ml of water at 35.03 is added to an unknown amount of water at 10.0 C and the final temperature of the mixture is 35.4 C , What was the mass of the water 10.0 C ?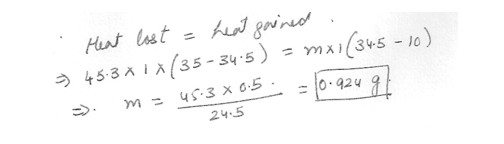
Physics Heat & Thermodynamics Level: High School
How many joules are required to raise the temperature of 65.7 ml of ethyl alcohol from 25.4 to 45.3 C ?