Physics Heat & Thermodynamics Level: High School
An amount of silver at 95.6 C is added to 85.0 ml of water at 45.0 C and the final temperature of the water is 59.4 C , What amount of silver was added ?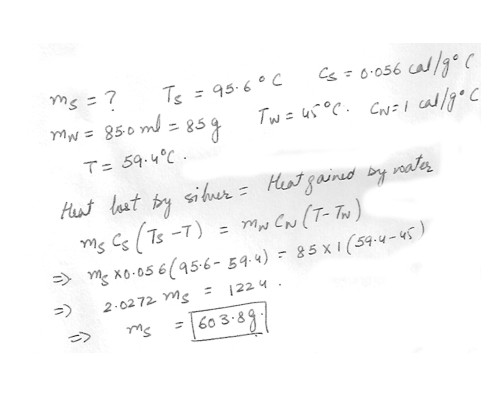
Physics Heat & Thermodynamics Level: High School
If 73.5 g of lead at 73.0 C is added to water at 25.0 C and the final temperature of the mixture is 53.0 C , what is the mass of the water ?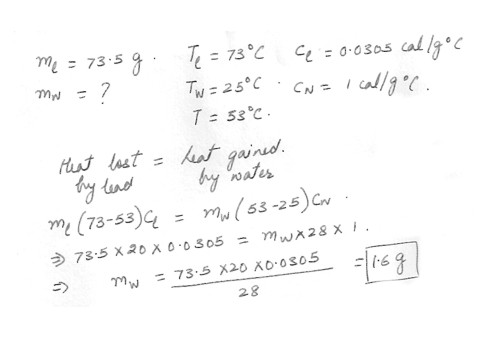
Physics Heat & Thermodynamics Level: High School
An ice tray containing 150 g of water at 30 degree C, is placed in a freezer at -5 degrees C . How much energy (J) must be removed from the water to lower its temperature to the freezing point ? How much total energy (J) must be removed from the water for it to reach the same temperature as the freezer ? ( The specific heat , s , for the liquid water is 4.18 J/g . degrees C and for water ice is 2.05 J/g . degree C ; the heat of fusion for water is 334 J/g and the heat of vaporization is 2256 J/g ).
Physics Heat & Thermodynamics Level: High School
A one cubic centimeter cujbe of iron and one cubic centimeter of water are both heated to 100 degrees C . Each is transferred separately into beakers each containing 100 grams of water at 10 degree C . Explain which beaker of water would reach a higher temperature , the one to which iron had been transferred or the one to which the water had been transferred .
Physics Heat & Thermodynamics Level: High School
What is the ratio of the average kinetic energies of the two types of molecule ?
Physics Heat & Thermodynamics Level: High School
How many helium molecules are there in the tank if it is filled at 20 degree C to a gauge pressure of 12.7 atm ?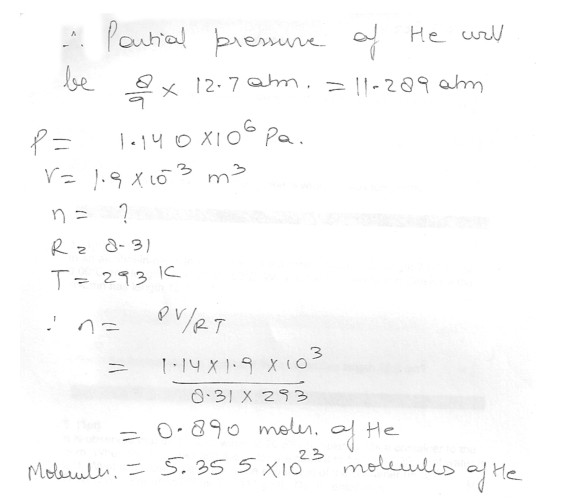
Physics Heat & Thermodynamics Level: High School
A scuba tank has a volume of 3800 cm^3.For every deep dives , the tank is filled with 50 percent (by volume ) pure oxygen and 50 percent pure helium . How many oxygen molecules are there in the tank if it is filled at 20 degreeC to a gauge pressure of 12.7 atm ?
Physics Heat & Thermodynamics Level: High School
What would be the rms speed of a protein of molecular mass 59900 u at 36.5 degree C ?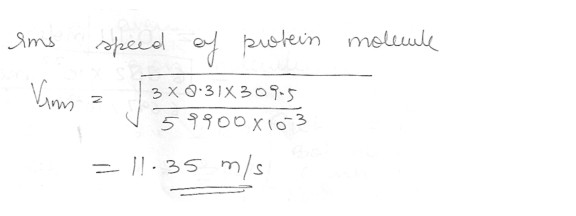
Physics Heat & Thermodynamics Level: High School
Calculate the approximate rms speed of an amino acid whose molecular mass is 95.0 u in a living cell at 36.5 degree C ?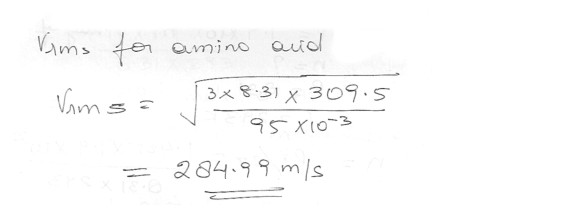
Physics Heat & Thermodynamics Level: High School
Calculate the mass speed of helium atoms near the surface of the sun at a temperature of about 6450 K .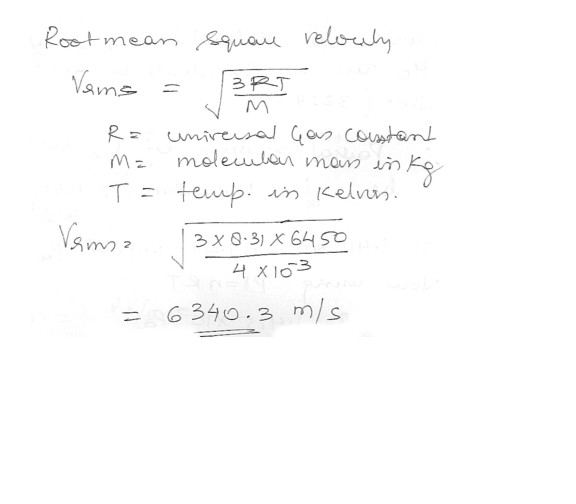
Physics Heat & Thermodynamics Level: High School
A tire is filled with air at 16.2 degreeC ato a gauge pressure of 221 kPa. If the tire reaches a temperature of 38.4 degree C . What fraction of the original air must be removed if the original pressure of 221 KPa is to be maintained ?
Physics Heat & Thermodynamics Level: High School
A storage tank contains 21.7 kg of nitrogen (N2) at an absolute pressure of 3.90 atm . What will the pressure be if the nitrogen is replaced by an equal mass of CO2 ?
Physics Heat & Thermodynamics Level: High School
What percent error is made in each if a person forgets to change degree C to K ?Do not enter units , and enter the size of the error as a positive number .

Physics Heat & Thermodynamics Level: High School
Typical temperatures in the interior of the Earth and Sun are about 3800 degree C and 1.45 x 10^7 degree C , respectively . What are these temperatures in Kelvin ? Do not enter units .
Physics Heat & Thermodynamics Level: High School
The pendulum in a grandfather clock is made of brass and keeps perfect time at 18.1 degreee C . How much time is gained or lost in a year if the clock is kept at 24.5 degree C ?Assume the frequency dependence on length for a simple pendulum applies. Enter a positive number for a gain , a negative number for a loss .

