Physics Heat & Thermodynamics Level: High School
Explain in less than 50-words why there is a minimum temperature (absolute zero) but no maximum temperature ?Physics Heat & Thermodynamics Level: High School
The dew point temperature of a saturated air parcel that is rising will:a. Decrease.
b. Increase.
c. Decrease and then increase.
d. Increase and then decrease.
e. Remain the same.
Physics Heat & Thermodynamics Level: High School
Saturation of an air parcel occurs when it has :a. 100% relative humidity.
b. 100% by volume of water vapor.
c. A temperature above dew point temperature.
d. A temperature equals to dew point temperature.
e. Both a and d.
f. None of the above.
Physics Heat & Thermodynamics Level: High School
A system for which the change in entropy of one part of the system is negative is a -------- process .a. Unnatural.
b. Natural .
c. Reversible.
d. Irreversible.
e. Insufficient data.
Physics Heat & Thermodynamics Level: High School
Three moles of an ideal gas are contained in a 30m^3 vessel at a pressure of 3.50 x 10^5 N/m^2 . The temperature of the gas is :a. 1.26 x 10^5 K
b. 4.21 x 10^4 K
c. 833 K
d. 4.17 x 10^-1 K
e. 2.52 x 10^5 K

Physics Heat & Thermodynamics Level: High School
An ideal gas undergoes an adiabatic compression . If its original volume and temperature were 0.5 m^3 and 373 K, What is its final temperature if its final volume is 1.0 m^3 ?a. 63 K
b. 186 K
c. 235 K
d. 373 K
e. 273 K

Physics Heat & Thermodynamics Level: High School
The efficiency of any heat engine :a. equals that of a carnot engine with the same intake and exhaust temperature .
b. equals W/Qinput .
c. depends only on its intake temperature .
d. depends only on it exhaust temperature .
e. equals that of a reversible heat engine with the same intake and exhasust temperature .
Physics Heat & Thermodynamics Level: High School
In order to apply the first Law of Thermodynamics in a situation, which of the following must be true ?a. The process must be irreversible
b. The system must be in thermodynamic equilibrium throughout the entire process.
c. The system need only be in thermodynamic equilibrium in its final and initial states.
d. The process must be adiabatic or at least partially so.
e. The process must be isentropic.
Physics Heat & Thermodynamics Level: High School
A 57.1 kg ice –skater moving at 6.44 m/s glides to a stop. Assuming the ice is at 0 degreeC and that 59.2 percent of the heat generated by friction is absorbed by the ice , how much ice melts ?
Physics Heat & Thermodynamics Level: High School
A hot iron horseshoe (mass = 0.440 kg ) which has just been forged , is dropped into 1.76 L of water in a 0.200 kg iron pot initially at 20.4 degree C , if the final equilibrium temperature is 25.6 degreeC , calculate the initial temperature of the hot horseshoe ( in Celsius )
Physics Heat & Thermodynamics Level: High School
At a crime scene, the forensic investigator notes that 11.3 –g lead bullet that was stopped in a door frame apparently melted completely on impact. Assuming the bullet was fired at room temperature of 20 degreeC , What does the investigator calculate the minimum muzzle velocity of gun was ?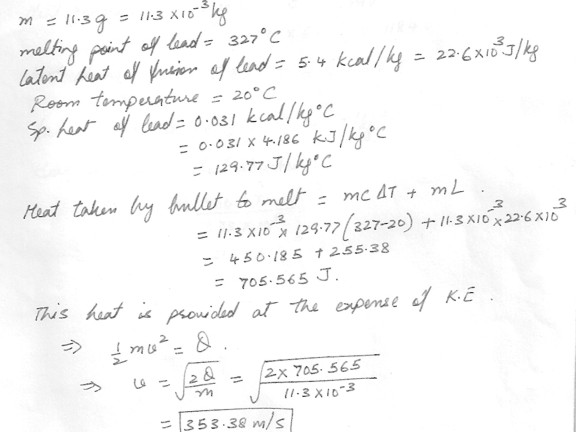
Physics Heat & Thermodynamics Level: High School
A brass plug is to be placed in a ring made of iron. At room temperature , the diameter of the plug is 8.791 cm and that of the inside of the ring is 8.786cm. They must both be brought to what common temperature (in degreeC) in order to fit ?
Physics Heat & Thermodynamics Level: High School
The temperature of an ideal gas is increased from 136 degreeC to 368 degreeC while the volume and the number of mole stay constant . By what factor does the pressure change ?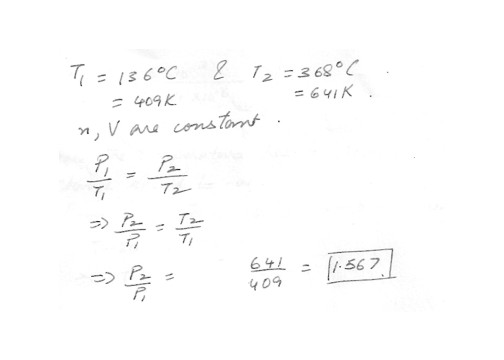
Physics Heat & Thermodynamics Level: High School
To make a secure fit , rivets that are larger than the rivet hole are often used and the rivet is cooled (usually in dry ice) before it is placed in the hole . A steel rivet 1.818 cm in diameter is to be placed in a hole 1.814 cm in diameter . To what temperature (on the Celsius scale) must the rivet be cooled if it is to fit in the hole (at 20.0 degreeC) ?
Physics Heat & Thermodynamics Level: High School
0.1 kg of water at 92.0 degree C is poured into an insulated cup containing 0.246 kg of ice initially at 0 degreeC . Calculate the mass of liquid when the system reaches thermal equilibrium .
