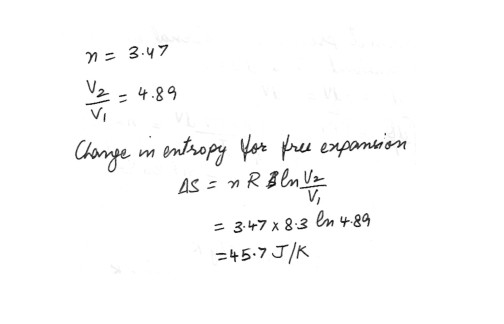Physics Heat & Thermodynamics Level: High School
Calorimetry
Thirty grams of ice , initially at -10 degreeC , are put into 300 gms of water , originally at 75 degree C , contained in a glass beaker . The final temperature of the system is 64.5 degreeC . Ignore any heat loss from the glass beaker . Find the heat of fusion of the ice .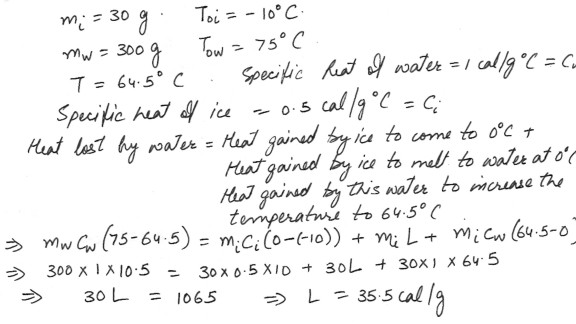
Physics Heat & Thermodynamics Level: High School
7Density can be defined as molar mass, MM, divided by molar volume , V .(a) determine (derivative of d / derivative of T) for an ideal gas in terms of MM , V , and p
Interpret your response to (a), especially with respect to the sign expected for the values of the derivative .

Physics Heat & Thermodynamics Level: High School
A desperate chem 321 student needed a sample of a pure gas for a series of experiments on gas expansions. But alas no tank of pure gases were available , undaunted , he decided to use Br2, and to work at temperatures above its boiling point. She first obtained Br2 vapor to fill a 5.000 L glass bulb at 150.0 degrees Celsius and 1.00 atm, the performed the following experiments.Expansion of final volume of 10.000 L against a constant pressure of .300 atm, holding the temperature constant, Calculate change in U and H, w, and q.
Reversible, isothermal, expansion to a final volume of 10.000L. calculate change in E and H, w,q
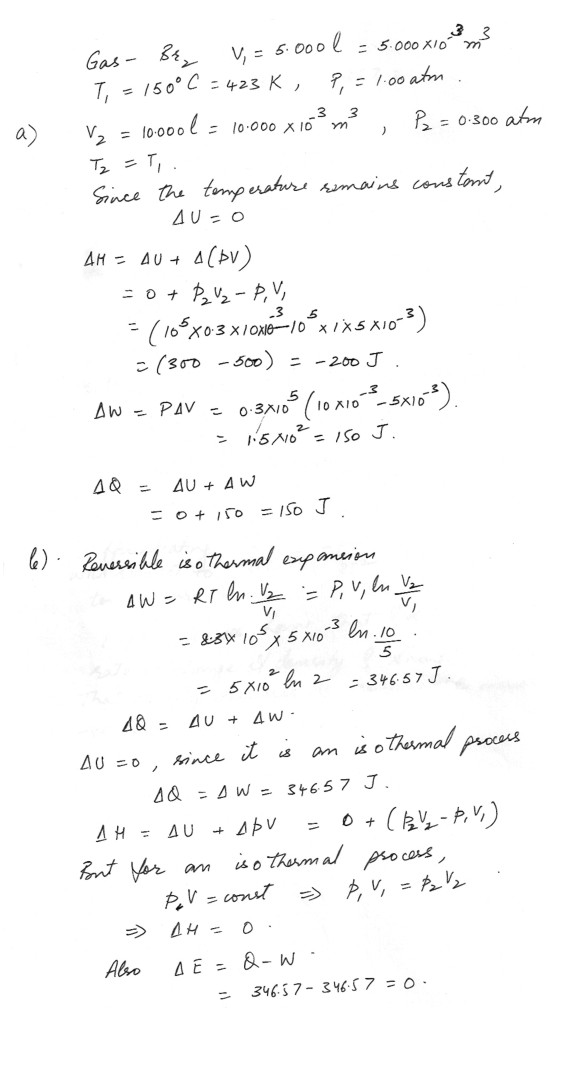
Physics Heat & Thermodynamics Level: High School
The heat capacity of aluminum can be described by : Cp = 20.67 + (12.38 x 10 to the negative 3) T J/mol K(a) How much heat is necessary to heat 10.00 g of AL from 25 degrees Celsius to 535 degrees Celsius .
(b) list a least two assumptions implicit in your calculations of part (a)

Physics Heat & Thermodynamics Level: High School
Give definitions of Cv and Cp by appropriate partial derivatives and operationally (ie , how do they work and what do they describe ?)
Physics Heat & Thermodynamics Level: High School
The Dieterici equation of state for real (non-ideal) gases may be written asP = RT/V-x (-y/rv)/ (e) for n = 1 mole
What characteristics of the gas molecules would you expect the Dieterici constant x and y to be related to ? Explain in terms of the assumptions of the kinetic molecular theory ?

Physics Heat & Thermodynamics Level: High School
A volume of 50.0 liters of oxygen at 10 C and an absolute pressure of 1.88 atm is compressed to 36.6 liters , and at the same time the temperature is raised to 80 C . What will the absolute pressure be now ?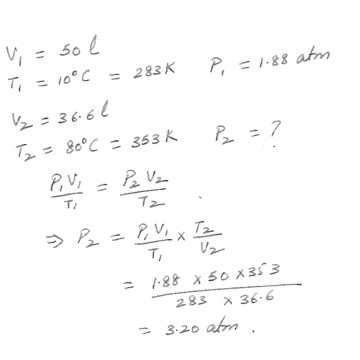
Physics Heat & Thermodynamics Level: High School
Calorimetry
Calculate the latent heat of fusion of ice from the following data for ice at 0 C added to water in a calorimeter . (results to 3 sing figs).Specific heat of calorimeter 0.100 cal (gm C)
Mass of calorimeter 70.0 gm
Mass of calorimeter plus water 507.0 gm
Mass of ice added 169.0 gm
Initial temperature of plus calorimeter 40.0 C
Final temperature of the mixture 7.0 C

Physics Heat & Thermodynamics Level: High School
Thermal Expansion
A steel bar 115 mm long has a cross –sectional area of 145 mm2 . when its temperature is increased by 25 C , it will expand (lengthen)by a certain small amount , change (triangle ) L . What stretching force , F , would need to be applied to this same change in length , without any change in temperature . For steel Y = 20 x 10 (to the tenth power) N/m^2 .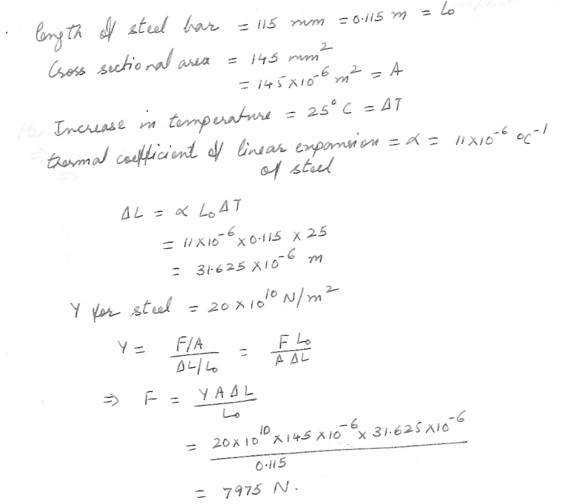
Physics Heat & Thermodynamics Level: High School
Carnot’s Engine
An engine operates at half its theoretical (carnot) efficiency between 545 C and 310 C. What is the efficiency of the engine ?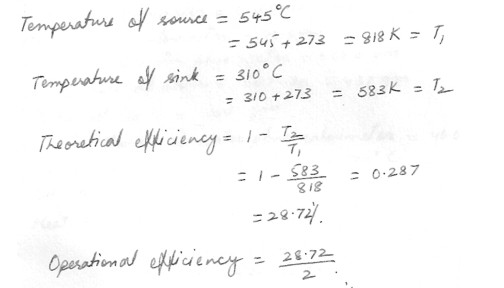
Physics Heat & Thermodynamics Level: High School
To maintain the temperature inside a house at 26 degree C , the power consumption of the electric baseboard heaters is 10 kW on a day when the outside temperature is -9 degrees C . At what rate does this house contribute to the increase in the entropy of the universe ? Answer in units of W/K .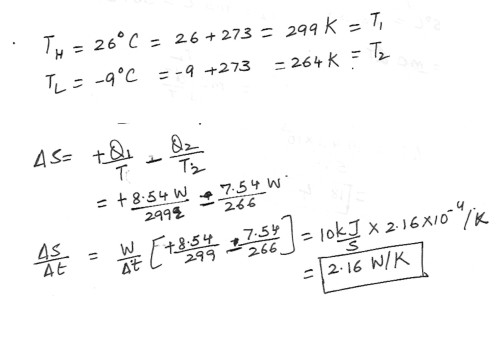
Physics Heat & Thermodynamics Level: High School
A refrigerator operator will Carnot efficiency between temperatures -4.8 degrees C and 33.3 degree C . What is the coefficient of performance ?
Physics Heat & Thermodynamics Level: High School
A heat pump has a coefficient of performance of 3.82 . If the heat pump absorbs 20cal of heat from the cold outdoors in each cycle , find the heat expelled to the warm indoors . Answer in units of cal .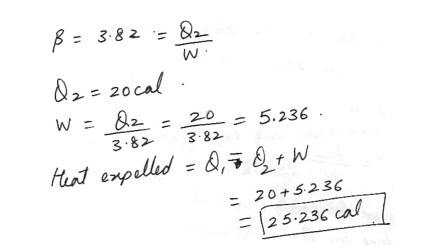
Physics Heat & Thermodynamics Level: High School
A mass of 1.67 kg of chilled water at 0 degreesC is placed in a freezer which is kept at -19.7 degrees C . The freezer works as a Carnot engine , cice = 2.09 kJ/kg x degreesC and seawater = 4.186 kJ/kg degree C . Approximately how much electric energy is needed to operate the compressor to cool this water to -19.7 degreeC if the room temperature is maintained at 29.2 degreeC ? Answer in units of kJ .
Physics Heat & Thermodynamics Level: High School
Calculate the change in entropy of 3.47 mol of an ideal gas that undergoes a free expansion to 4.89 times its initial volume . Answer in units of J/K .