Physics Heat & Thermodynamics Level: High School
By how much will a slab of concrete 18 m long contract when the temperature drops from 24 degreeC to -16 degreeC ? (The coefficient of linear thermal expansion for concrete is 12 x 10^-6 per degreeC .)0.17 cm
0.52 cm
0.86 cm
8.6 cm
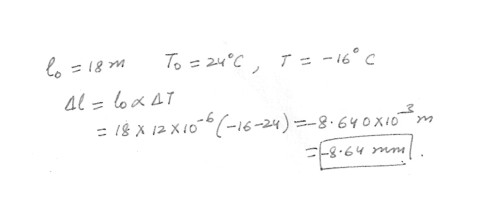
Physics Heat & Thermodynamics Level: High School
Calorimetry
A metal vessel with a heat capacity of 0.8 kjKL^-1 contains a mixture of 0.6 kg of ice and 0.4 kg of water in equilibrium at 0.0 degreeC . Into this vessel is bubbled 0.1 kg of steam at 100 degreeC . When thermal equilibrium is re-established , it is found that all the ice is melted and that the vessel contains only water at temperature theta . Assuming the container is so well lagged that it exchanges a negligible amount of energy with the surroundings , calculate the value of theta .
Physics Heat & Thermodynamics Level: High School
A perfectly insulated calorimeter contains 500 ml of water at 30 degrees Celsius and 45 g o ice at (zero) degrees Celsius . Determine the final temperature of the system . Keep units cosistant .
Physics Heat & Thermodynamics Level: High School
The brick wall k = 1.2 W/m . degree C of a building has dimensions of 2.8 m by 16 m and is 8 cm thick . How much heat flows through the wall in a 9.7 h period when the average inside and outside temperature are , respectively , 32 degree C and . 4 degree C ? Answer in units of MI .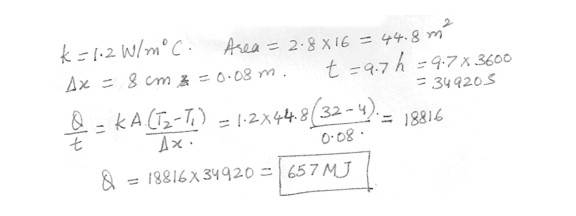
Physics Heat & Thermodynamics Level: High School
Specific heat of water = 1 cal/(g.degree C) . How much heat is required to vaporize a (n) 6 g ice cube initially at 0 degreeC ? The latent heat of fusion of the ice is 80 cal / g and the latent heat of vaporization of water is 540 cal/g . Answer in units of cal .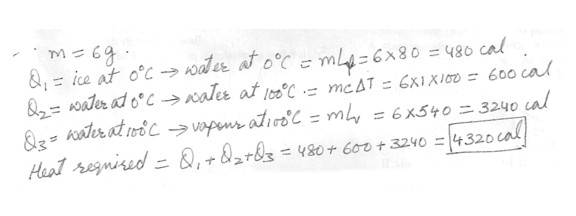
Physics Heat & Thermodynamics Level: High School
Given:lf = 79500 cal/kg,
lv = 540000 cal/kg,
c = 1000 cal/kg. degree C.
1 kg of ice at 0 degree C is converted to steam at 100 degree C.
How much heat is needed ? Answer in units of kcal.

Physics Heat & Thermodynamics Level: High School
latent heat of vaporization of water
2.26 x 10^6 J/kg .
A person who eats 2953 cal each day consumes 12361.3 J of energy in a day . How much water at 100 degree C could that much energy vaporize ? Answer in units of g .
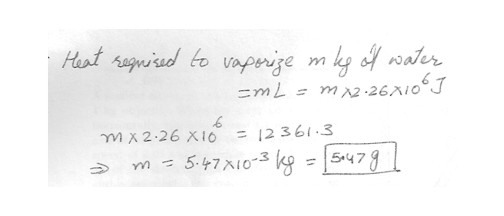
Physics Heat & Thermodynamics Level: High School
Given: specific heat of water
1000 cal/kg degree C.
A mass of 3 kg of water is raised from 36 degree C to the boiling point 100 degree C . How much heat is required . Answer in units of cal .
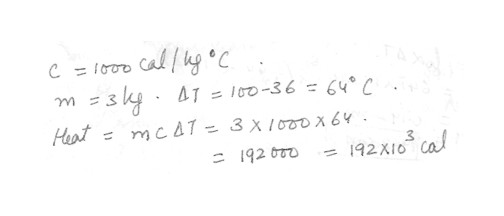
Physics Heat & Thermodynamics Level: High School
Styrofoam container used as a picnic cooler contains a block of ice at 0 degree C . If 439 g of ice metals in 1 hour , how much heat energy per second is passing through the walls of the container ? (The heat of fusion of ice is 333000 J/kg). Answer in units of W .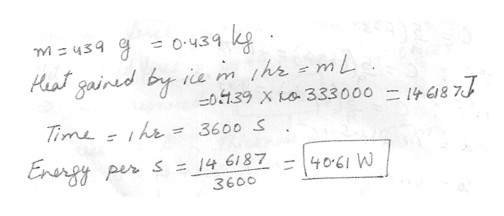
Physics Heat & Thermodynamics Level: High School
The potential at a certain distance from a point charge is 600 Volts , and the electric field strength is 200 N/C (Newton per Coloumb)What is the distance to the point charge ?
What is the magnitude of the charge ?
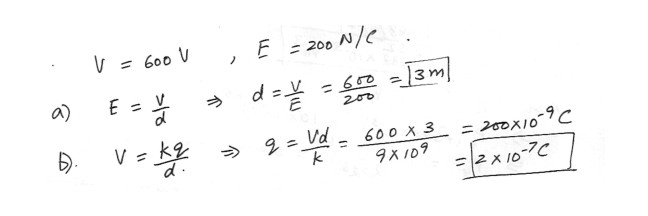
Physics Heat & Thermodynamics Level: High School
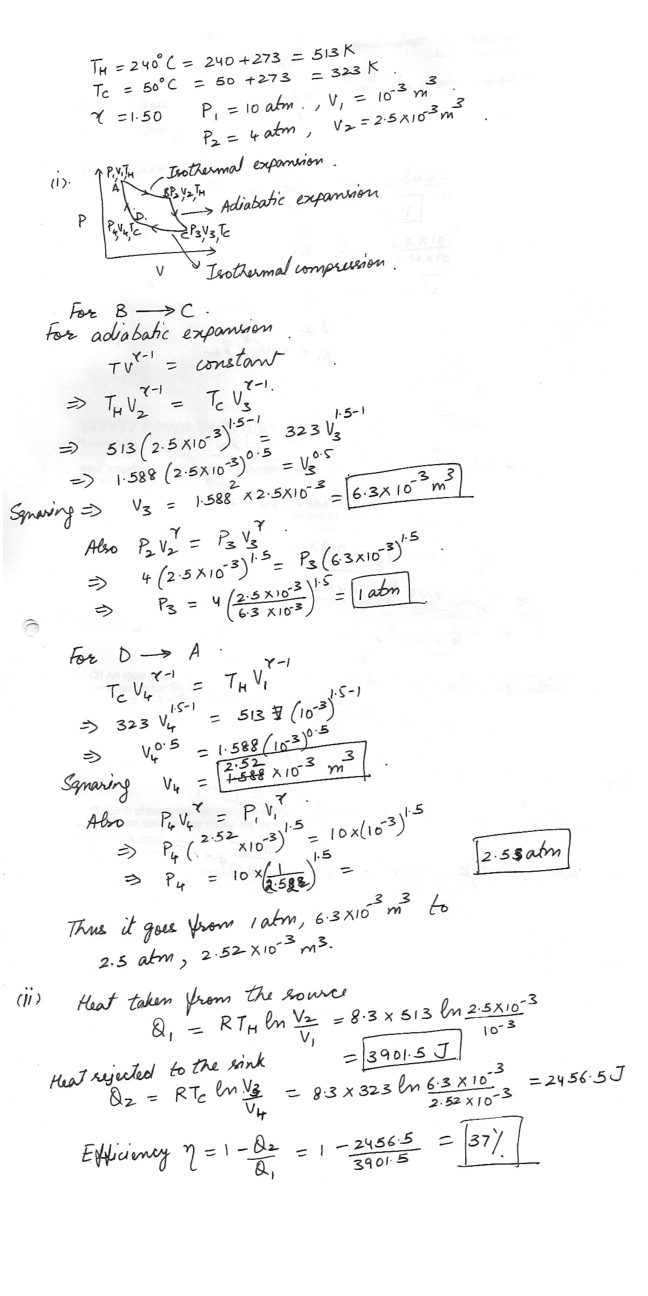
An ideal gas for which g = 1.50 is used as the working substances in a cylinder undergoing a Carnot cycle between temperatures TH = 240 degreeC and TC = 50 degreeC . At TH it is expanded from a pressure of 10 atm and a volume of 10-3 m3 to a pressure of 4 atm and a volume of 2.5 . 10-3 m3 .Between what limits of pressure and volume does it operate at TC ?
Calculate the heat taken from the source and the heat rejected by the sink in each cycle , and the efficiency , assuming perfect reversibility .
Physics Heat & Thermodynamics Level: High School
A refrigerator operates in a cyclic and reversible manner between 0 and 27 degreeC . How much work must be done in freezing 1L of water ? Calculate the coefficient of performance given that the talent heat of fusion of water is 334.72 J per g .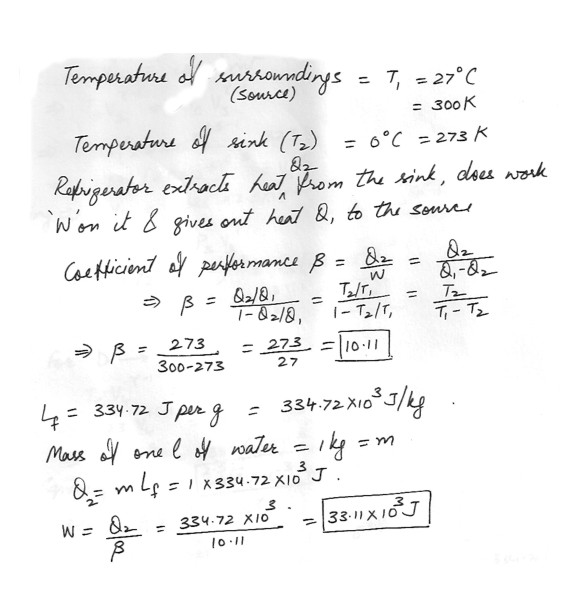
Physics Heat & Thermodynamics Level: High School
Latent heat of fusion of water 334000 J/kg
Year’s ago , a block of ice with a mass of about 21 kg was used daily in a home icebox , The temperature of the ice was 0.0 degree C , when delivered . As it melted how much heat did a block of ice that size absorb ? Answer in units of J .Physics Heat & Thermodynamics Level: High School
Which two temperature changes are equivalent ?a. 1 degree F = 1 degree C
b. 1 degree C = 1 K
c. 1K = 1 degree F
Physics Heat & Thermodynamics Level: High School
Consider a flat steel plate with a hole through its center . When the plates temperature is decreased, the hole will .a . contract only if it takes up more than half the plate’s surface area.
b . expand if it takes up less than half the plate’s surface area.
c . contract.
d . expand.
