Physics Heat & Thermodynamics Level: High School
A solid substance that has a latent heat of fusion 21.6 kJ/kg melts at a temperature 302 degree C . Calculate the change in entropy that occurs when 0.483 kg of this substance is melted . Answer in units of J/K .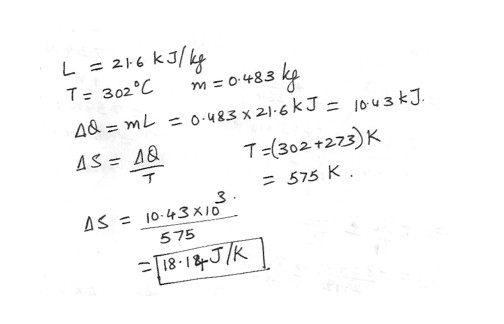
Physics Heat & Thermodynamics Level: High School
Calculate the change in entropy of 149 g of water heated slowly from 8.5 degreeC to 91.5 degree C . Answer in units of J/K .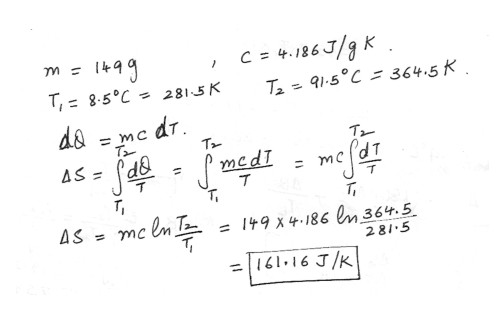
Physics Heat & Thermodynamics Level: High School
n mol of an ideal gas are compressed isothermally from an initial volume V1 to a final volume V2 .What is the change in entrophy
Change in S=n*k*In (v2/v1)
Change in S=n* (Cv/T)
Change in S=n*Cv*Integral of dT/T
Change in S=n*R*In(V2/V1)
Change in S=n*R*T*In(V2/V1)

Physics Heat & Thermodynamics Level: High School
On a hot day , a Florida home is kept cool by an air conditioner . The outside temperature is 36.6 degreeC and the interior of the home is 15.2 degreeC . If 152 kJ/h of heat is removed from the house . What is the minimum power that must be provided to the air-conditioner ? Answer in units of kJ/h .
Physics Heat & Thermodynamics Level: High School
The radius of the sun is 6.96 x 10^8 m and the total power output from its surface is 2.51 x 10^26w .Assuming that sun acts like a black body , calculate its surface temperature .
What is the maximum wavelength of radiation from the sun ?
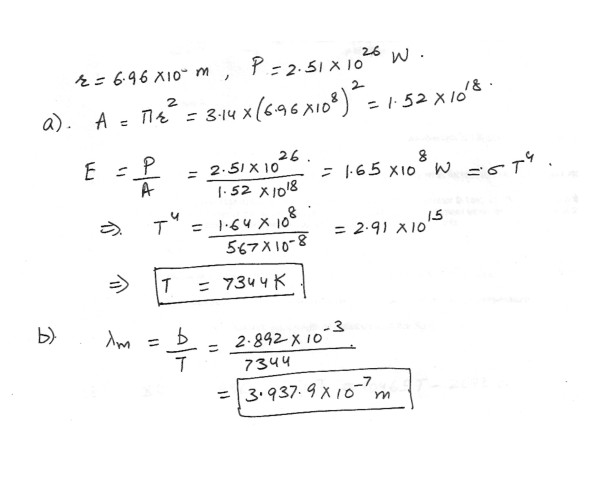
Physics Heat & Thermodynamics Level: High School
(a) A heat source has its radiation maximum when the wavelength is 1.8 micro m . Calculate the temperature of the heat source .
(b)If the absolute temperature of a black of body is doubled , by what factor does the total power increase ?
(c)A tungsten filament of an incandescent bulb is a wire diameter 0.1 cm . length 10 cm and acts like a black body . Calculate the power radiated from the surface of the filament when its temperature is 2000 degreeK . Neglect any radiation from the two contact (cross –sectional area )points .

Physics Heat & Thermodynamics Level: High School
(a) How much energy is required to change 40g of ice from ice at -10 degreeC to steam at 110 degreeC ?A combination of (0.25 kg of water at 20degreeC), (0.4kg of aluminium at 26 degreeC) and (0.1 kg of copper at 100 degreeC) is mixed in an insulated container and allowed to come to thermal equilibrium . Ignoring any energy transfer to or from the container , determine the final temperature of the ‘mixture’

Physics Heat & Thermodynamics Level: High School
(a) A bar of gold is in thermal contact with a bar of silver of the same length and area . The compound bar is well lagged with one end maintained at 80 degreeC while the opposite end is maintained at 30 degreeC . When the energy transfer reaches a steady state , what is the temperature of the junction ?
(b) The temperature of a silver bar rises by 10 degreeC when it absorbs 1.23 kJ of heat energy. If the mass of the bar is 525 g find the specific heat capacity of silver .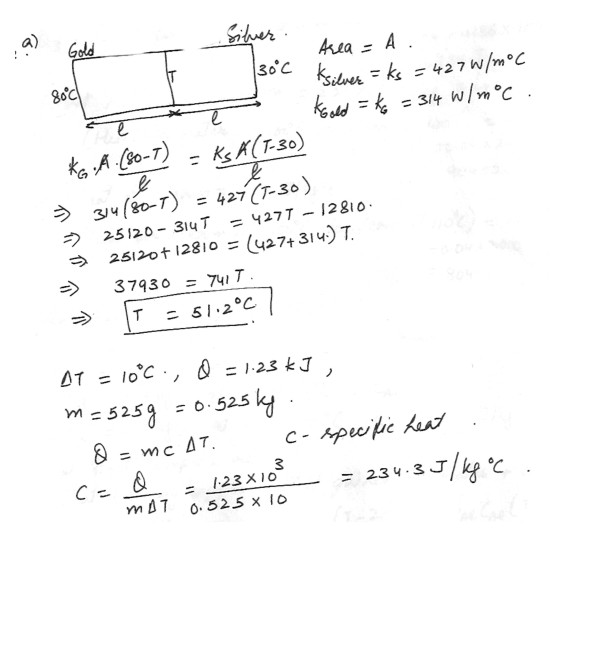
Physics Heat & Thermodynamics Level: High School
(a) A student eats a meal of 2000 kcal (kilocalories ) . He wishes to do an equivalent amount of work in the gym by lifting a 50 kg barbell . How many times must he raise the barbell to expend this amount of energy assuming that he raised it from ground level to head level a distance of 2m and that he regains no energy when he lowers the barbell to the floor ?(b)A glass window pane has an area of 3m^2 and a thickness of 0.6 mm . If the temperature difference between its face is 25 degreeC , what is the rate of energy transfer by conduction through the window ? (Thermal conductivity of glass is 0.8 W/m degree C .)

Physics Heat & Thermodynamics Level: High School
Aluminum has a positive coefficient of thermal expansion . Consider a round hole that has been drilled in a large sheet of Aluminum . As the temperature increases and the surrounding metal expands , the hole diameter will:Decrease
Increase
Remain constant
Depends how much metal surrounds the hole
Physics Heat & Thermodynamics Level: High School
How much heat is added 500 g of water to raise its temperature from 15degreeC to 85 degreeC ?35 j
3.5 x 10^4 j
1.5 x 10^5 j
1.5 x 10^8 j
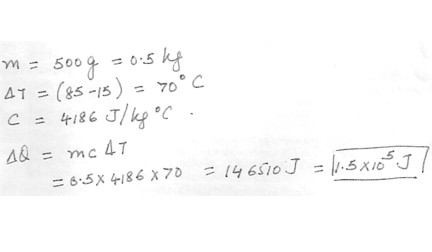
Physics Heat & Thermodynamics Level: High School
which of the following would correctly convert a Fahrenheit temperature (F) to a Kelvin (K) value ?K = (5/9) (F-32) + 273
K = (9/5) (F-32) + 273
K = (5/9) (F-32)
K = (9/5) F + 273
K = (5/9) F -273
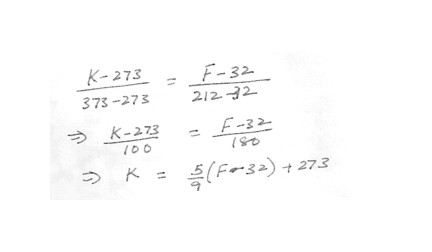
Physics Heat & Thermodynamics Level: High School
A steel cable spanning a river is 195.946 m long when the temperature is 20 degreeC . What will be its length when the temperature drops to 0 degree C ? (The coefficient of thermal) expand of steel is195.997m
195.903m
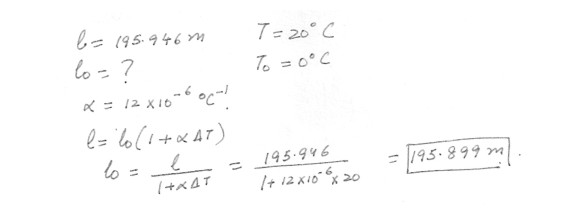
Physics Heat & Thermodynamics Level: High School
Consider a flat steel plate with a hole through its center , When the plate’s temperature is decreased , the hole willExpand if it takes up less than half the plates surface area .
Contract only if it takes up more than the half the plate’s surface area .
Expand
Contract
Physics Heat & Thermodynamics Level: High School
Describe the anomalous thermal expansion property of water and its significance .
Temperature Near freezing , water has a negative expansion coefficient which means it expands as it cools and freezes . Since ice is less dense than liquid water , it floats and stays on top of lakes and oceans . This has allowed aquatic life to survive UNDER the ice even during very cold times .Temperature Near freezing , water has a positive expansion coefficient which means it expands as it cools and freezes . Since ice is more dense than the liquid water , it floats and stays on top of lakes and oceans . This has allowed aquatic life to survive UNDER the ice even during very cold times .

