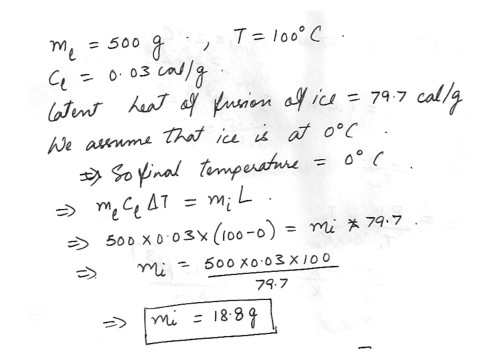Physics Heat & Thermodynamics Level: High School
(a) If 0.8 mol of a gas in a container occupies a volume of 14.01 at a temperature of 1.2 atm, What is the temperature of the gas (in K) ?(b) The container is fitted with piston so that the volume can change . When the gas is heated at constant pressure , it expands to a volume of 19.01 What is the temperature of the gas (in K) ?
(c) The volume is fixed at 19.01 and the gas is heated at constant volume until its temperature is 333.0 K . What is the pressure (in atm) of the gas now ?
Physics Heat & Thermodynamics Level: High School
(a) Lake Erie contains roughly 3.90 E + 11 m^3 of water. How much heat is required to raise the temperature of that volume of water from 10.8 degreeC to 13.0 degreeC ?(b) How many years would it take to supply this amount of heat by using the full output of a 1150 MW electric power plant ? Do not enter unit

Physics Heat & Thermodynamics Level: High School
A class of 10 students taking an exam has a power output per student of 124 W. Assume that the initial temperature of the room is 18.0 degreeC and that its dimensions are 5.90 m by 14.5 by 3.00m . What is the temperature (in degree C , do not enter units) of the room at the end of 61.0 min if all the heat remains in the air in the room and none is added by an outside source ? The specific heat of air is 834 J/kg*degreeC , and its density is about 1.32 E-3g/cm^-3 .
Physics Heat & Thermodynamics Level: High School
A 110 g ice cube at 0 degreeC is placed in 628 g of water at 25.2 degreeC . What is the final temperature of the mixture ? Do not enter units .
Physics Heat & Thermodynamics Level: High School
How much heat is required to change a 46.8 g ice cube from ice at -10.8 degreeC to steam at 115 degreeC ?
Physics Heat & Thermodynamics Level: High School
A 71.4 kg weight-watcher wishes to climb a mountain to work off the equivalent of large piece of chocolate cake rated at 470 (food) Calories . How high must the person climb ? ( 1 food calorie = 10^3 calories)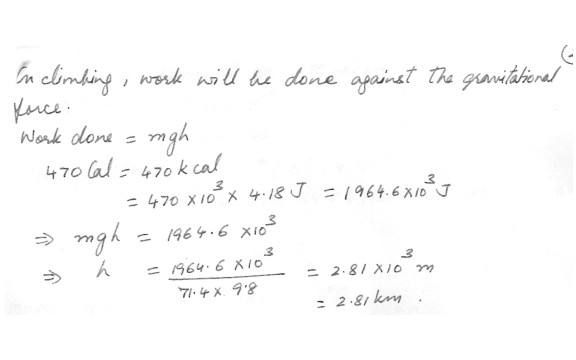
Physics Heat & Thermodynamics Level: High School
An air bubble has a volume of 1.49 cm^3 when it is released by a submarine 104 m below the surface of a lake . What is the volume of the bubble when it reaches the surface ? Assume that the temperature of the air in the bubble remains constant during ascent ?
Physics Heat & Thermodynamics Level: High School
Gas is confined in a tank at a pressure of 11.8 atm and a temperature of 14.1 degreeC . If half of the gas is withdrawn and the temperature is raised to 69.8 degreeC , What is the new pressure in the tank ?
Physics Heat & Thermodynamics Level: High School
You picked up an iron shot-put ball a track meet and were surprised at how heavy it was , it had a mass of 7.25 kg . The iron had the same temperature as the atmosphere (25 celsius) . How many calories must the iron ball absorb to reach its melting point and melt ?(specific heat of iron = 0.11 cal/g Celsius, melting point of iron = 1530 celsius , heat of fusion of iron = 22.0 cal/g)

Physics Heat & Thermodynamics Level: High School
How many kg of Al is experience the same temperature rise as 3 kg of Cu when the same amount of heat is added to each one ?[Clue to solve : 0.05 kg Al @ 20 degree C . Add 200J of heat . What is the final temperature ]
Physics Heat & Thermodynamics Level: High School
8 meters long steel rails (coefficient of linear equation = 12 x 10^-6) are laid end to end in winter when the temperature is -10 degree C . How much space should be left between them to allow for expansion in the summer , when the temperature could reach 50 degree C ? 64.5 degree C ?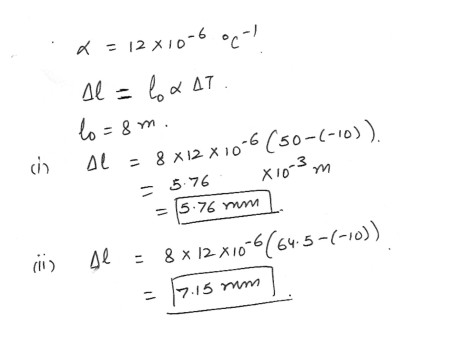
Physics Heat & Thermodynamics Level: High School
Calorimetry
An aluminum calorie meter of mass 50 g contains 95 g of a mixture of water and ice at 0 degrees C . When 100 g of aluminum at 100 degreesC is dropped into the mixture , the temperature rises to 5 degree C . The specific heat of aluminum is 0.22 cal/g - “C . Find the mass of the ice originally present .
Physics Heat & Thermodynamics Level: High School
Calorimetry
An ideal gas is contained within a volume of 2 ft^3 when the pressure is 137 atmospheres and the temperature is 27 degreeC . What volume would this gas occupy if it were allowed to expand to atmosphere pressure at a temperature of 50 degree C .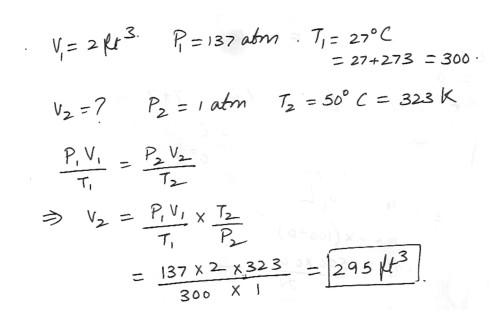
Physics Heat & Thermodynamics Level: High School
Calorimetry
When 2 pounds of brass at 212 degrees F is dropped into 5 lbs of water at 35 degrees F , the resulting temperature is 41.2 degrees F . Ignore the effect of the container . Find the specific heat of the brass .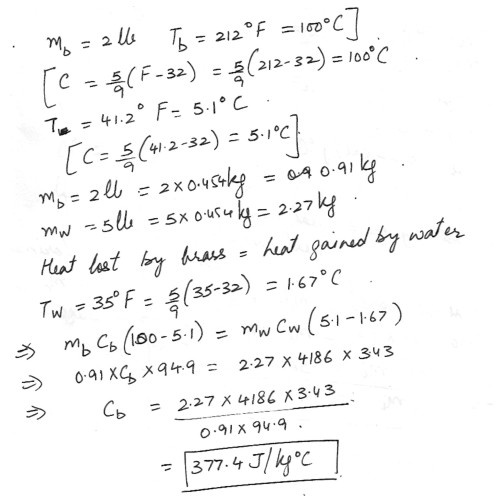
Physics Heat & Thermodynamics Level: High School
Calorimetry
500 g of lead at a temperature of 100 degreeC is poured into a hole in a large block of ice . The specific heat of lead is –“C 0.03 cal/g-“ . How much ice is melted ?