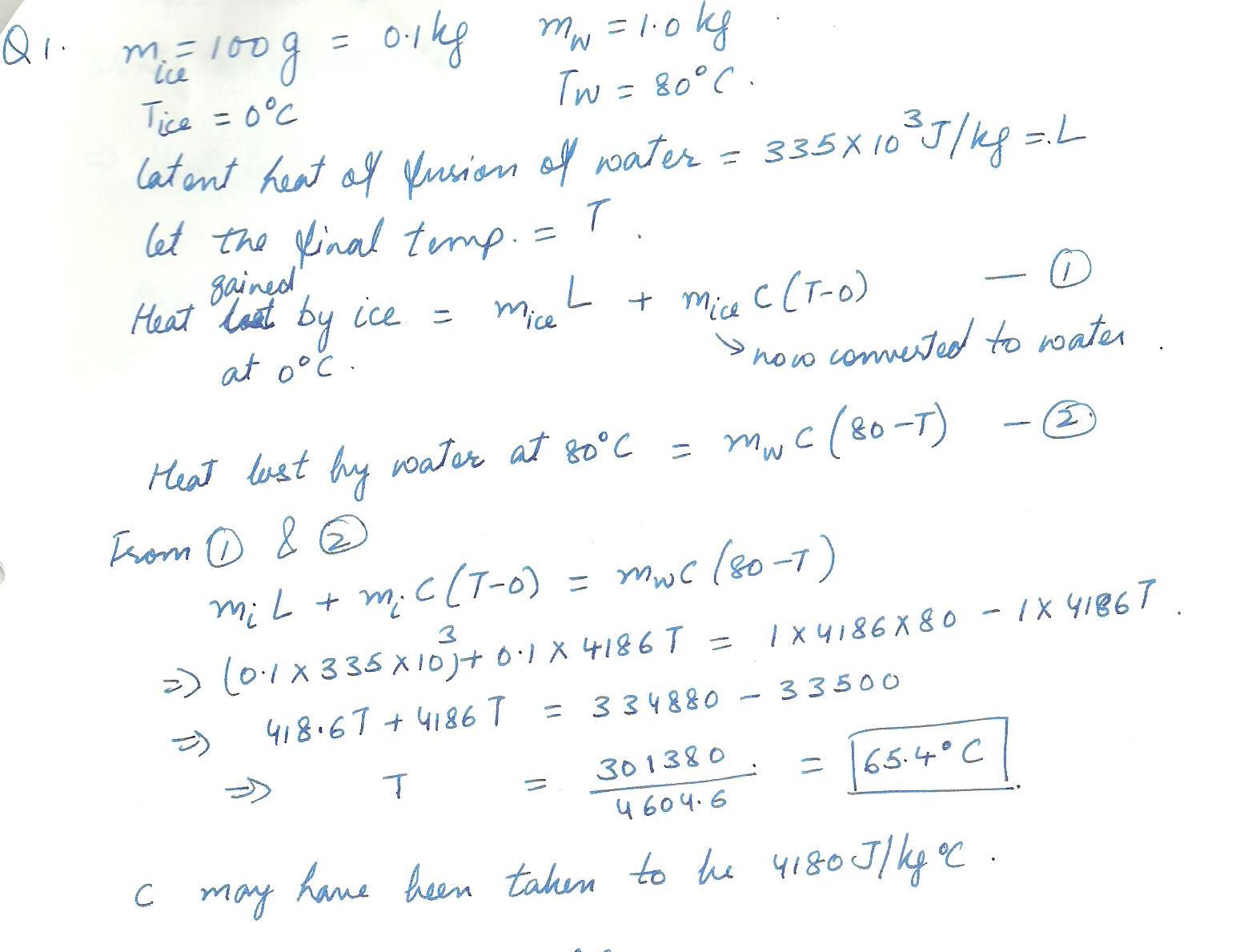Physics Heat & Thermodynamics Level: Misc Level
A 10 -kg iron ballis dropped onto a pavement from a height of 100 m.If half of the heat generated goes into warming the ball, find the temperature increase of the ball.(In SI units,the specific heat capacity of iron is 450 J/kg*OC.) Why is the answer the same for an iron ball of any mass?

Physics Heat & Thermodynamics Level: Misc Level
Calculate the latent heat of fusion of ice from the following data for ice at 0%C added to water in a calorimeter.(Give your result to three signicant figures).
a. Specifiic heat of the calorimeter 0.100 cal (gm%C)
b. Mass of the calorimeter 70.0 gm
c. Mass of calorimeter plus water 507.0 gm
d.Mass of ice added 169.0 gm
e.Initial temp of water plus calorimeter 40.0%C
f. Finial temp of the mixture 7.0% C
Lf=

Physics Heat & Thermodynamics Level: Misc Level
An engine operates at half its theoretical (carnot) efficiency between 545%C and 310% C. What is the efficiency engine? efficiency=
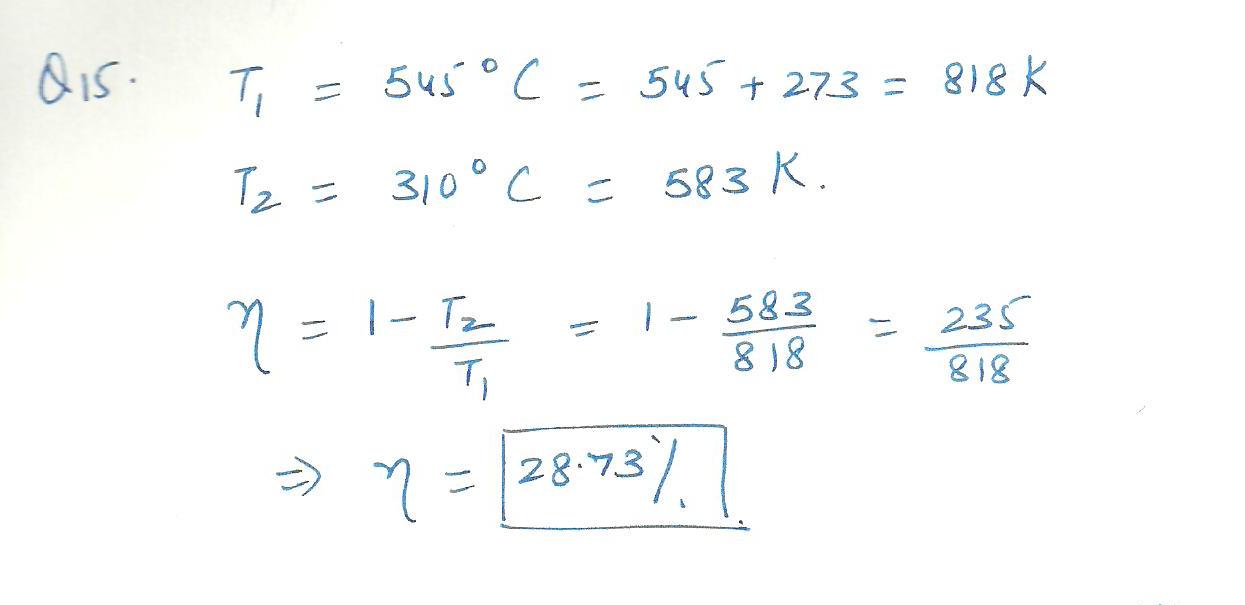
Physics Heat & Thermodynamics Level: Misc Level
An adiabatic process is one in which the system being considered
a. remains at constant temperature
b. remains at constant pressure
c. has no heat flow into or out of it during the process
d.underoes no change in internal energy
e. does no work, and has no work done on it
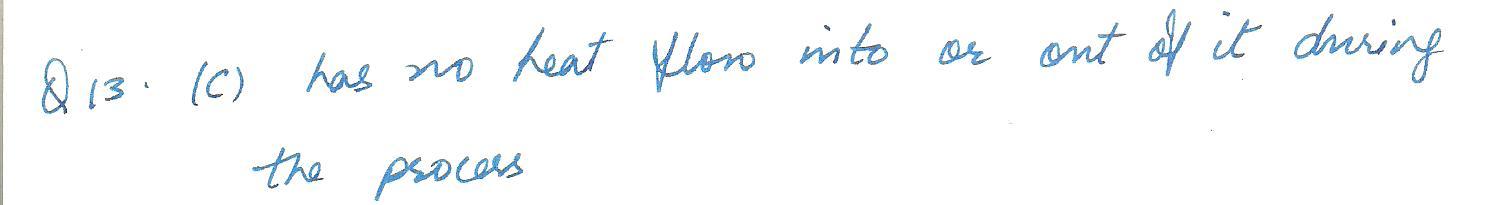
Physics Heat & Thermodynamics Level: Misc Level
A 1- kg chunk of ice at 0 degrees C melts, absorbing 80,000 cal of heat in the process. Which of the following beast describes what happens to this system?
a. increased entropy
b.lost entropy
c. entropy maintained constant
d.work converted to energy
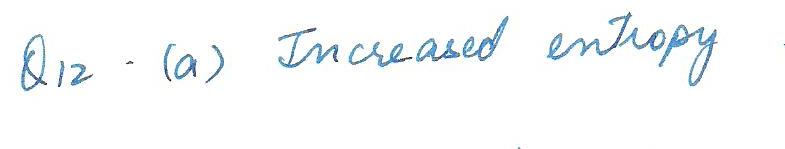
Physics Heat & Thermodynamics Level: Misc Level
The absolute temperature of an ideal gas is directly proportional to which of the following properties(when taken as an average) of the molecules of that gas?
a. speed
b.momentum
c.mass
d.kinetic energy
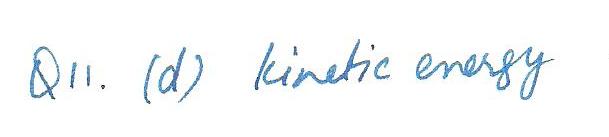
Physics Heat & Thermodynamics Level: Misc Level
In a cloud formation, water vapor turns into water droplets which get bigger and bigger until it rains. This will cause the temperature of the clouds to
a. get warmer
b. get cooler
c. be completely unaffected
d. there is no air in clouds
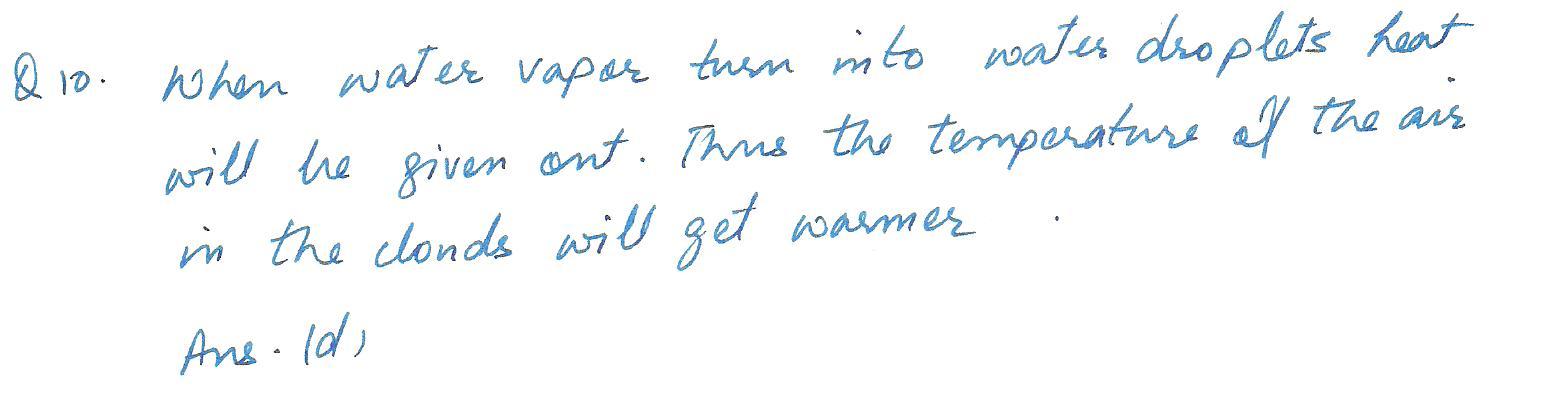
Physics Heat & Thermodynamics Level: Misc Level
Which of the following processes of heat transfer may take place in a vacuum?
a. Conduction
b.Convection
c.Radiation
d.Induction
e.None of the above
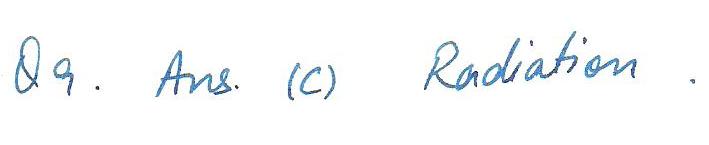
Physics Heat & Thermodynamics Level: Misc Level
A small immersion heater is rated at 3.0 E2 w. Estimate how long it will take to heat a cup of soup (assume this is 2.5 E 2 mL of water) from 22% C to 57% C.

Physics Heat & Thermodynamics Level: Misc Level
The coefficient of thermal conductivity has the unit:
a.j/(sk)
b.j/(mk)
c.w/(mk)
d.w/(sk)
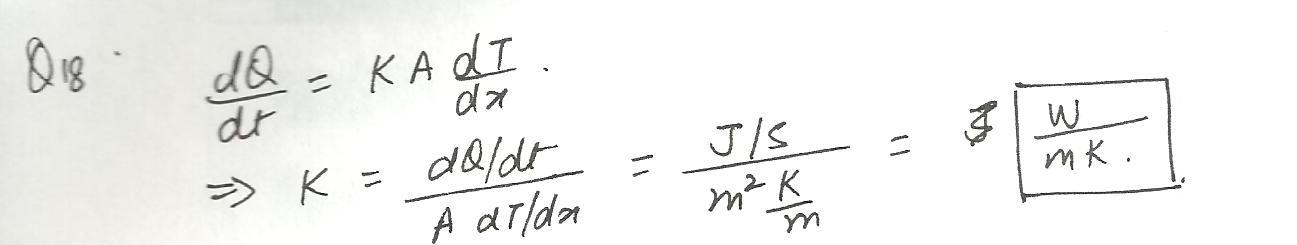
Physics Heat & Thermodynamics Level: Misc Level
petrol vapour is injected into the cylinder of a car engine when the piston is in its expanded position. The volume, pressure and temperature of the resulting gas-air mixture are 2cm 3 ,1-00x105 N m-3 and 20%C. The fuel mixture is then compressed adiatically to a volume of 38 cm3, Determine the pressure and temperature of the fuel mixture after compression has occurred.

Physics Heat & Thermodynamics Level: Misc Level
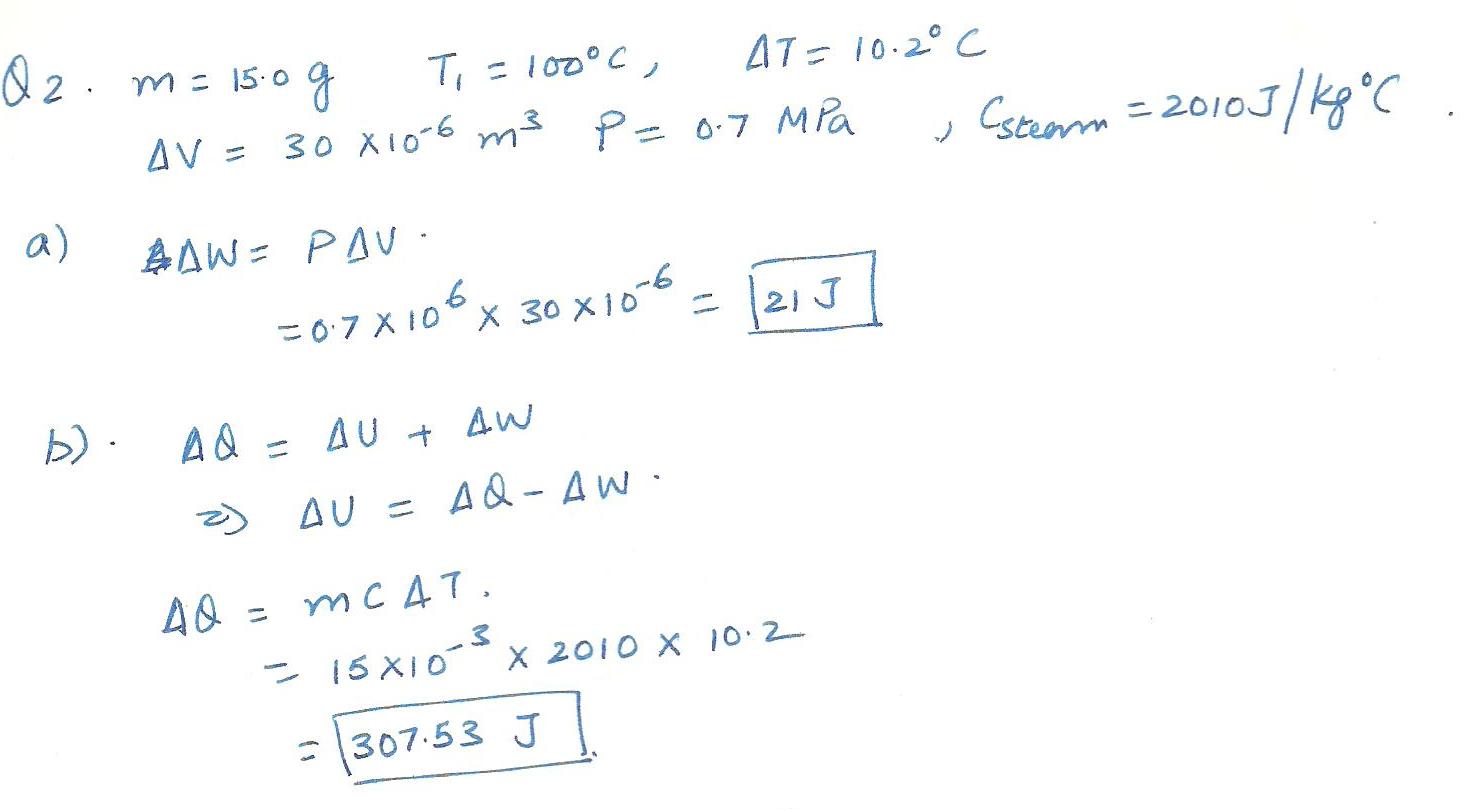
A cylinder closed off with a movable piston contaions 15.0 g of stcam at 100% C. The system is heated and its temperature increases by 10.2%C. as the steam expands 30 um3 at a constant pressure of 0.7 MPa.
Determine the:
a)work done by the steam
b)change in internal energy of the system.
Physics Heat & Thermodynamics Level: Misc Level
Calculate the maximum thermal efficiency of a heat engine operating with the same T(high) as a nuclear reactor . Use 20% celsius for T(low ).Explain why nuclear reactor has a low themal efficiency.

Physics Heat & Thermodynamics Level: Misc Level
Find the rate of energy flow through a copper block of cross sectional area 15 cm squared, and length 8.0 cm when a temperature difference of 30 degrees C is established accross the block. Repeat the calculation, assuming that the material is (b)a block of stagnant air with the given dimensions, and (c) a block of wood with the given dimensions.
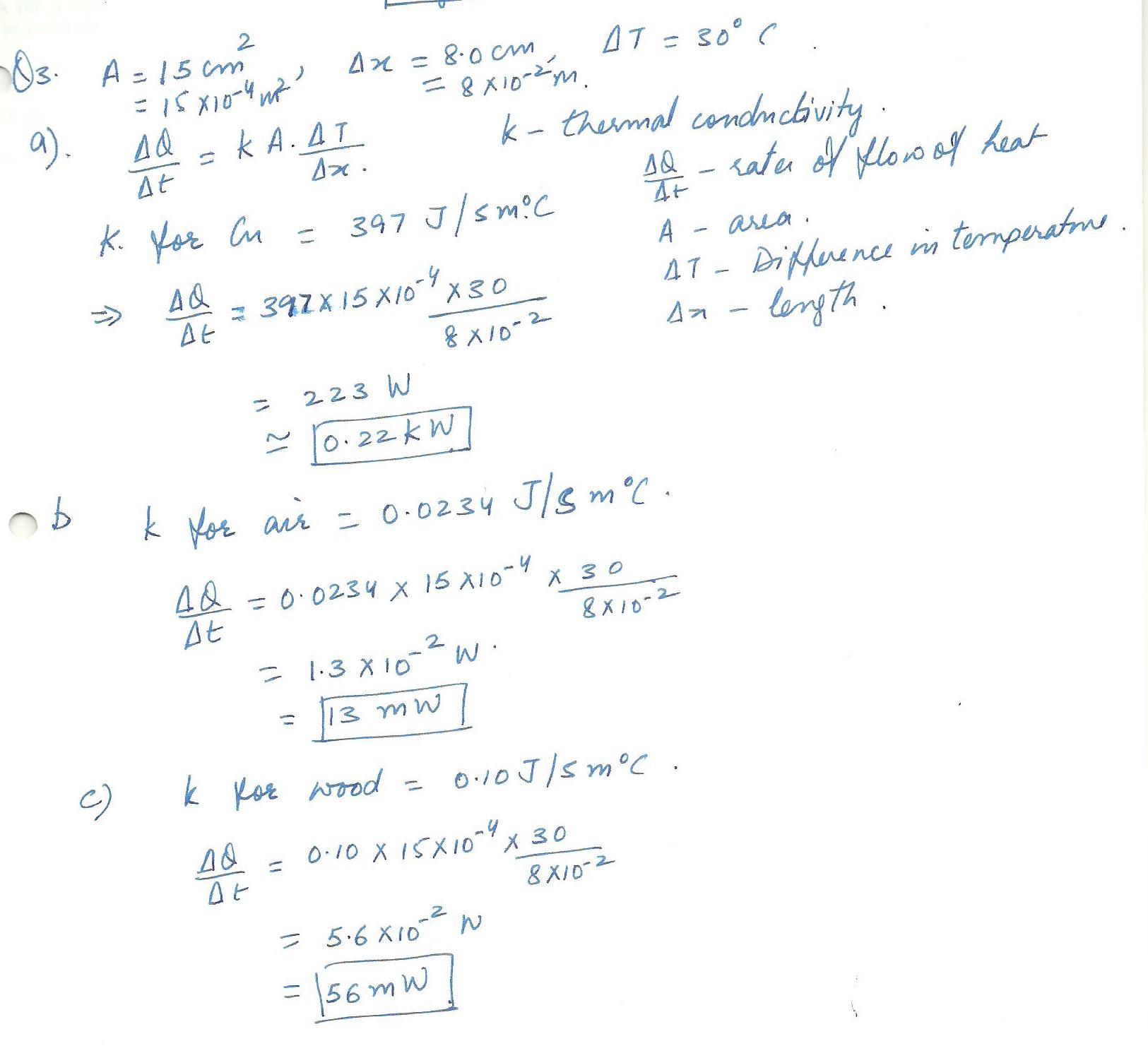
Physics Heat & Thermodynamics Level: Misc Level
A 100 g cube of ice at 0 degtrrs C, is dropped into 1.0 kg of water that was origionally at 80 degrees C.What is the final temperature of the water after the ice has melted?