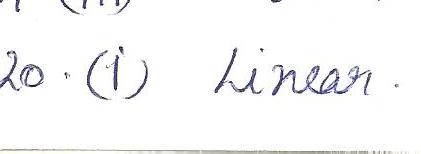Chemistry Inorganic Chemistry Level: Misc Level
What volume of 1.024 M H2SO4 is required for a complete reaction with 100.0ml of 0.8845 M NaOH??(acid base reaction produces salt and water)

Chemistry Inorganic Chemistry Level: Misc Level
When ammonium dichromate is heated, it decompeses to nirtogen gas, chromium oxide, and water.How many g of water will be produced when 1.0 g of ammonium dichromate is heated?
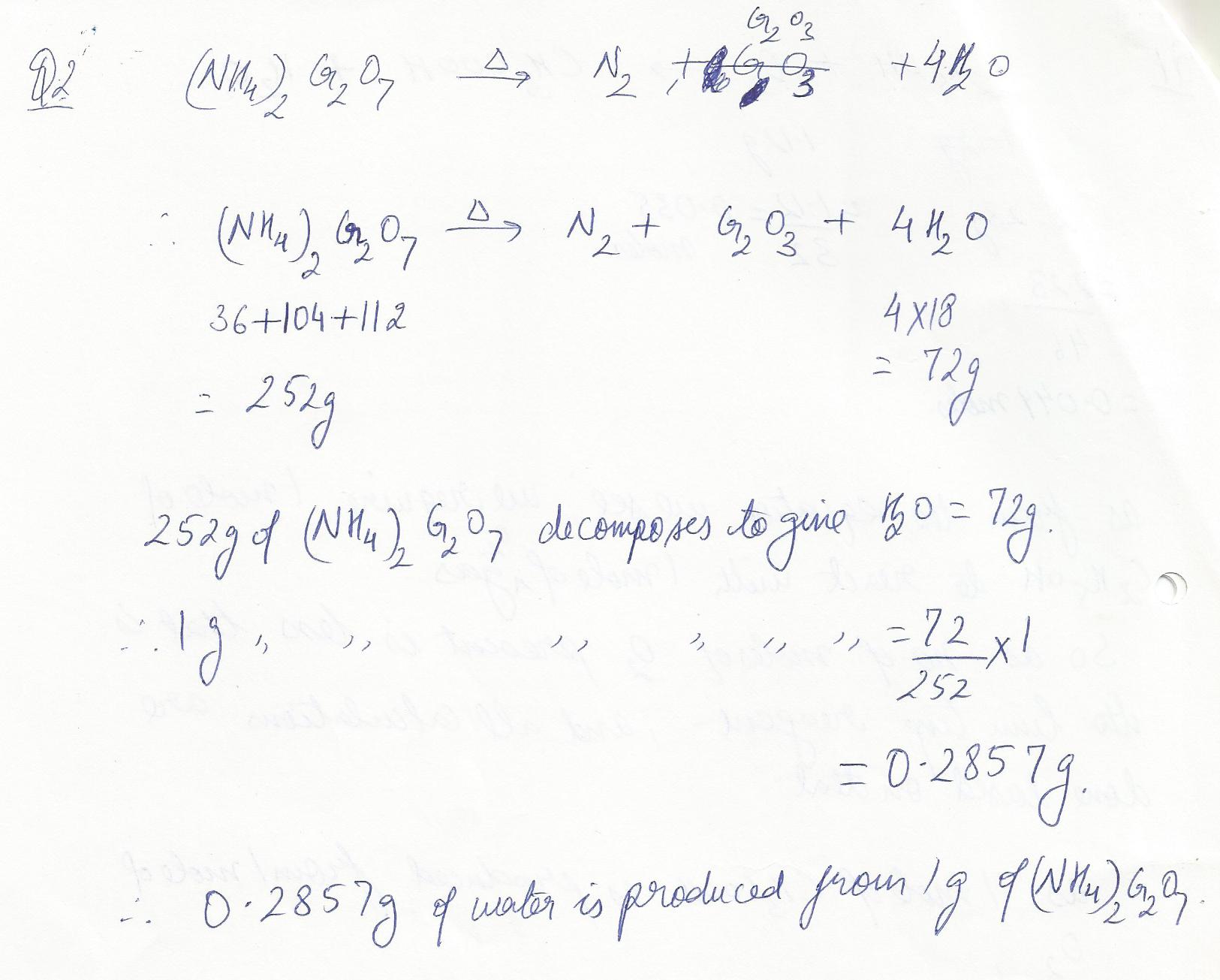
Chemistry Inorganic Chemistry Level: Misc Level
The rate determining step in a reaction is
a)the one with the highest activation energy
b) the one with the lowest activation energy.
c)thermodynamically favorable d)the fastest step.
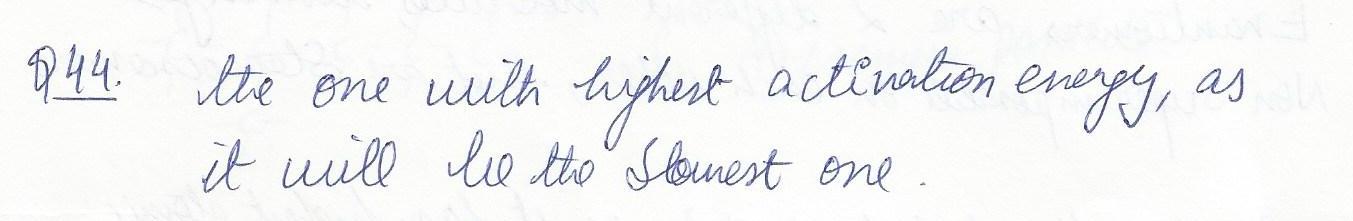
Chemistry Inorganic Chemistry Level: Misc Level
If a molecule is levorotatory,
it rotates plane polarized light in the counterclockwise direction.
It rotates plane polarized light in the clockwise direction.
it is optically inactive.
none of the above

Chemistry Inorganic Chemistry Level: Misc Level
A product of this acid-base reaction is; OH- + NH4+ --> ------ + NH3
NOH
H2O
NH5
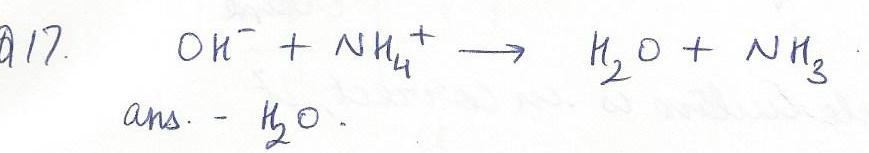
Chemistry Inorganic Chemistry Level: Misc Level
The nitronium ion,NO2+,is produced when nitric acid is mixed with sulfuric acid. Write a balanced equation for this reaction.
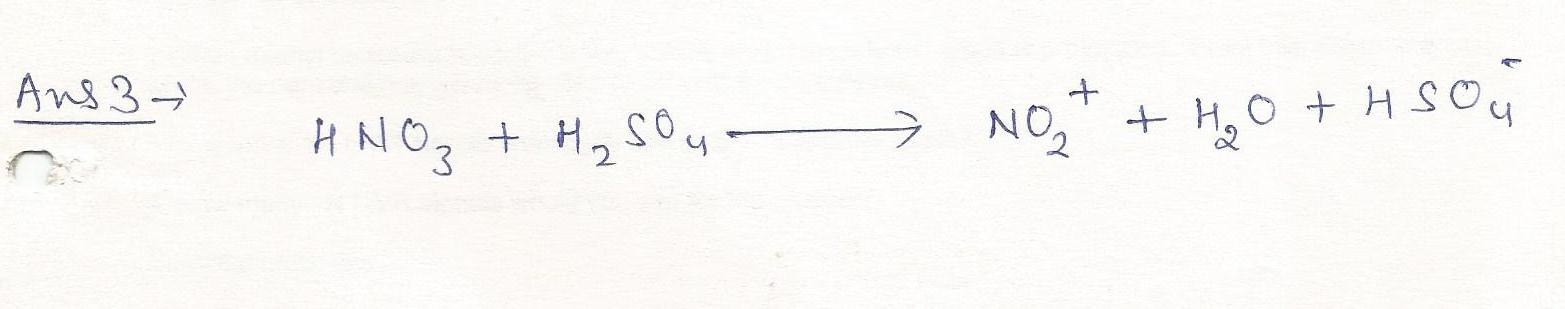
Chemistry Inorganic Chemistry Level: Misc Level
How many unpaired electrons are there for Cr (#24)?

Chemistry Inorganic Chemistry Level: Misc Level
Write the electronic configuration according to the general rules in the objective for Gd (#64).
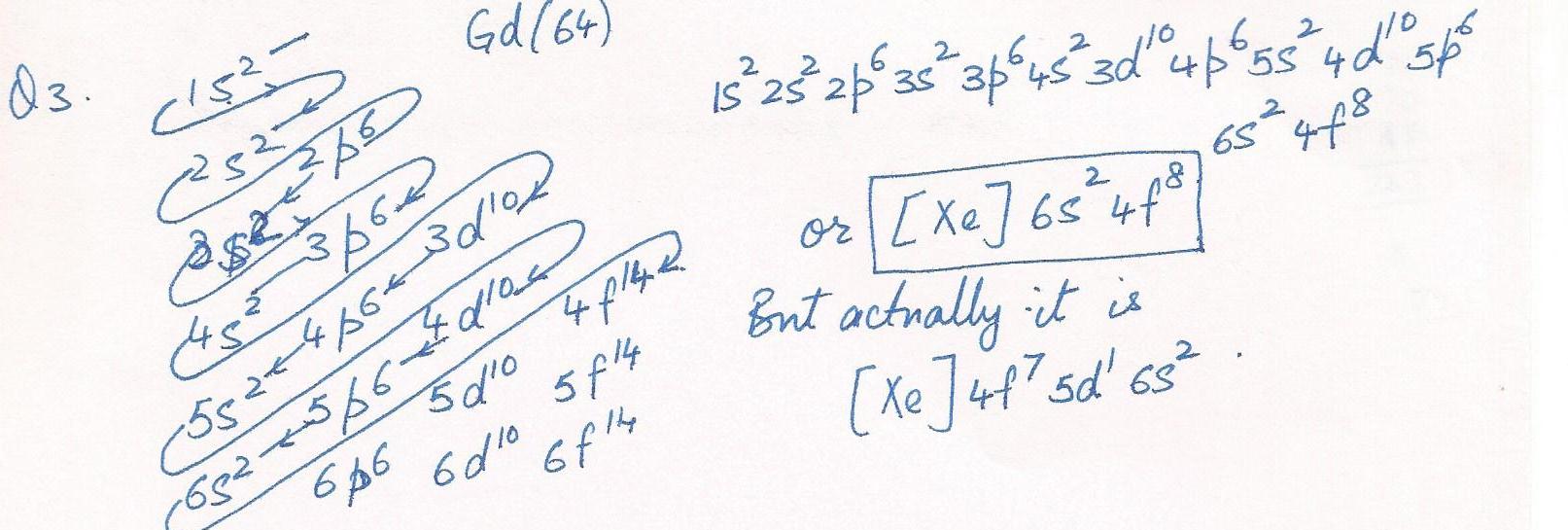
Chemistry Inorganic Chemistry Level: Misc Level
According to VSEPR theory, which of the following shapes is possible for a molecule with he molecular of AB2?
a. linear
b. bent
c.trigonal planar
d. both(a)&(b)
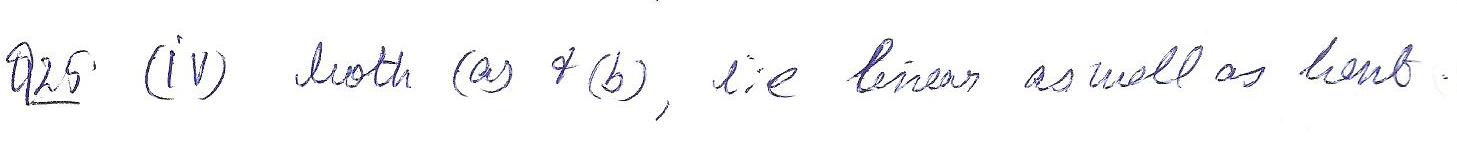
Chemistry Inorganic Chemistry Level: Misc Level
Name the following compound (NH4)3PO4
a.Ammonium Phosphite
b. Tri-ammonium Phospho tetraoxide
c. Ammonium Tri-phosphide
d.Ammonium Phosphate

Chemistry Inorganic Chemistry Level: Misc Level
Name the following compound (NH4)3PO4
a.Ammonium Phosphite
b. Tri-ammonium Phospho tetraoxide
c. Ammonium Tri-phosphide
d.Ammonium Phosphate

Chemistry Inorganic Chemistry Level: Misc Level
Which of the following molecules would most likely be classified as a tetrahedral shape?
a. HCI
b. CO
c. C4H8
d. CH4
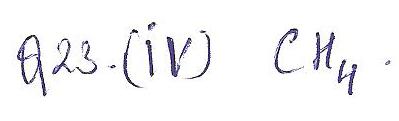
Chemistry Inorganic Chemistry Level: Misc Level
To draw a Lewis structure, you DO NOT need to know
a. the number of valence electrons for each atom
b. the types of atoms in the molecule
c. the number of atoms in the molecule
d. bond energies

Chemistry Inorganic Chemistry Level: Misc Level
One can predict the shape of a molecule by drawing its Lewis structure and
a. balancing the oxidatin numbers
b. accounting for nonbonded pairs of valence electrons
c. determing the empirical formula
d. identifying the presence of polyatomic ions
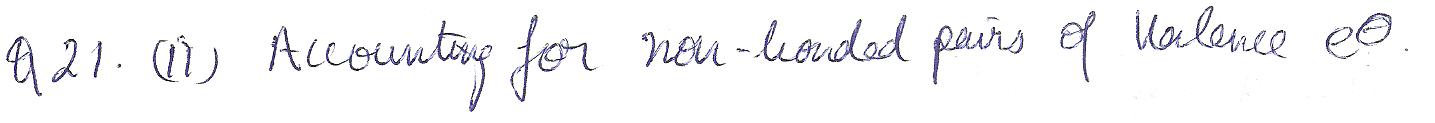
Chemistry Inorganic Chemistry Level: Misc Level
According to the VSEPR theory the molecular shape of CO2, carbon dioxide,is classified as
a.linear
b.bent
c. trigonal planar
d. trigonal pyramidal