Physics Modern Physics Level: Misc Level
Describe how the Bohr concept of the atom was able to explain the discrete sprctra for Hydrogen.Include stationary stares, emission, exited energy states. Diagrms can be used.

Physics Modern Physics Level: Misc Level
What is the difference between fission and fusion?

Physics Modern Physics Level: Misc Level
A beam of light, with intensity 63 W/m2 and polarization parallel to a y axis, is sent into a system of two polarizing sheets with polarizing directions at angles of 01=%52% and 02=90% to the y axis. What is the intensity of the light transmitted by the two -sheet system?
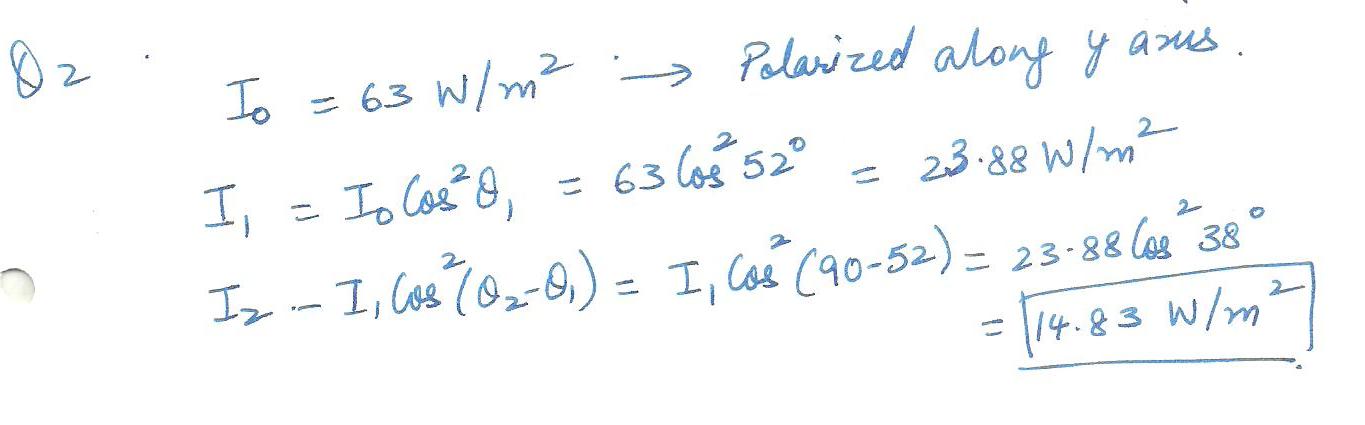
Physics Modern Physics Level: Misc Level
Initially unpolarized light is sent into a system of three polarizing sheets whose polarizing directions make angles of 01=36%,02=28%, and 03=52% with the direction of the y axis. What percentage of the light,s initial intensity is transmitted by the system?

Physics Modern Physics Level: Misc Level
An electron and proton are moving at the same speed, which has a higher de Broglie wavelength?
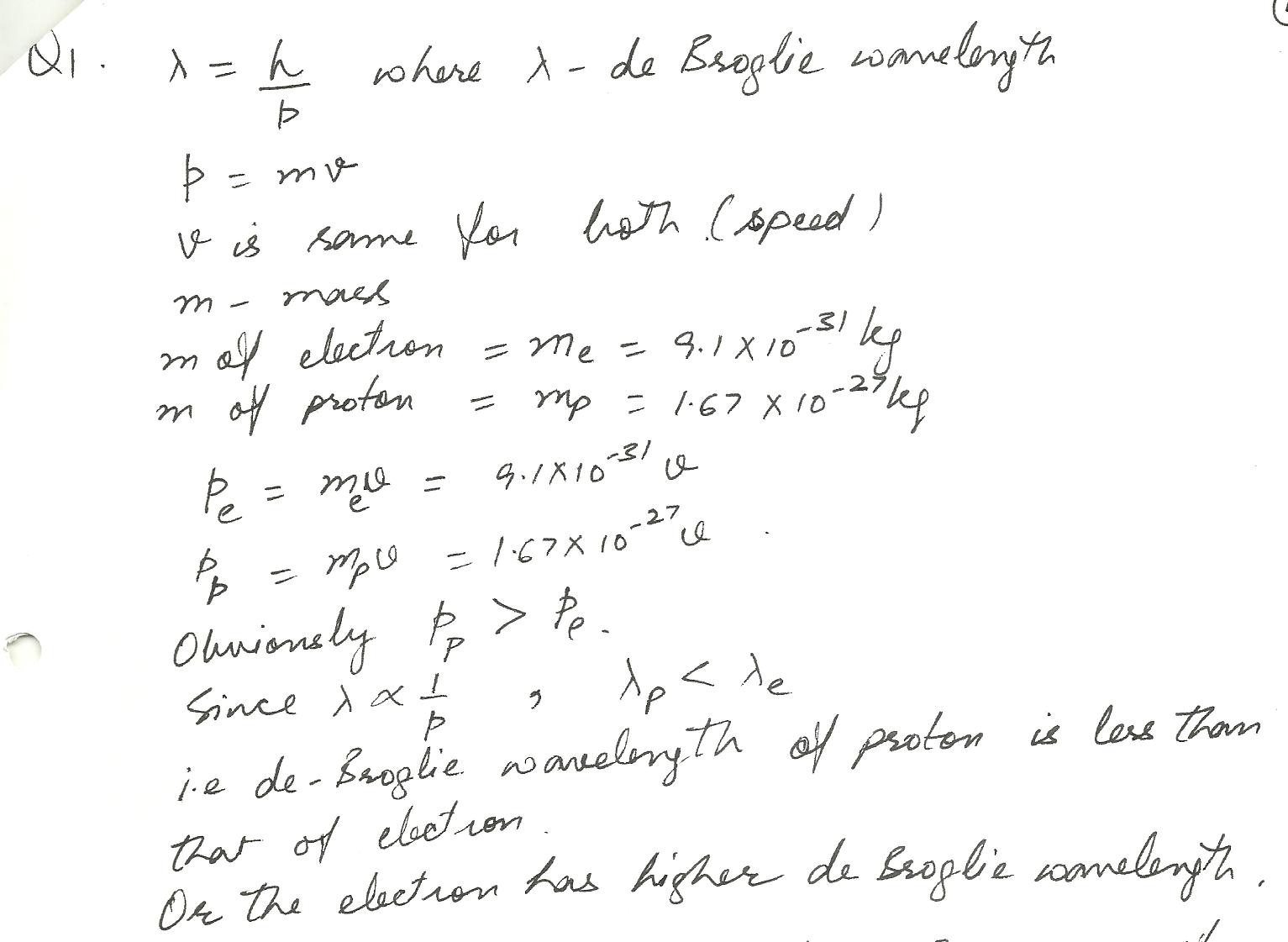
Physics Modern Physics Level: Misc Level
A hydrogen atom, treated as a point mass, is confined to an infinite one diamensional square well of width 1.0 nm.How much energy does it have to give up to fall from the level with n=2 to the lowest energy level?
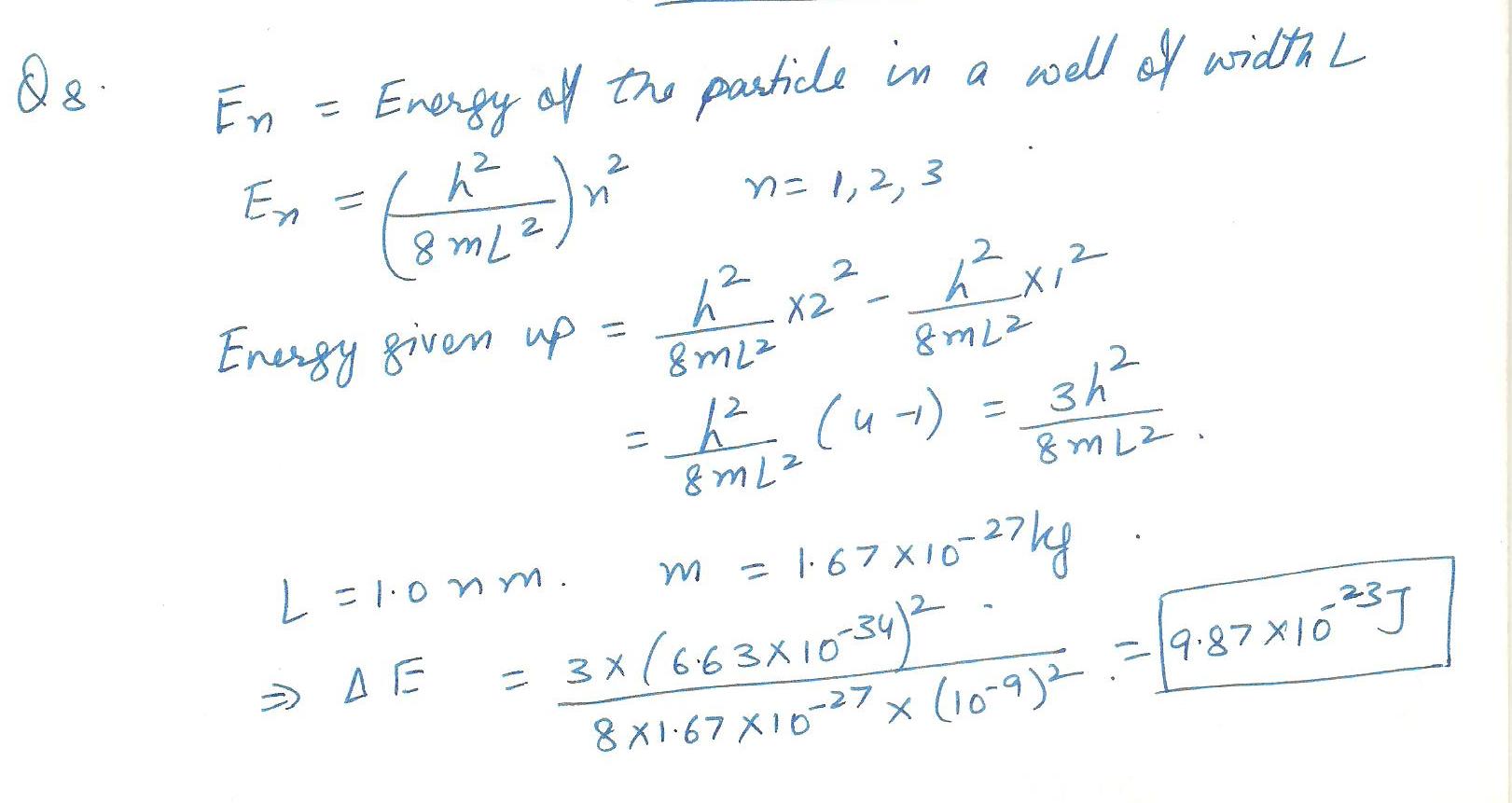
Physics Modern Physics Level: Misc Level
Calculate the minimum uncertainty in the speed of a ball of mass 500g that is known to be within 5 um of a certain point on a bat.
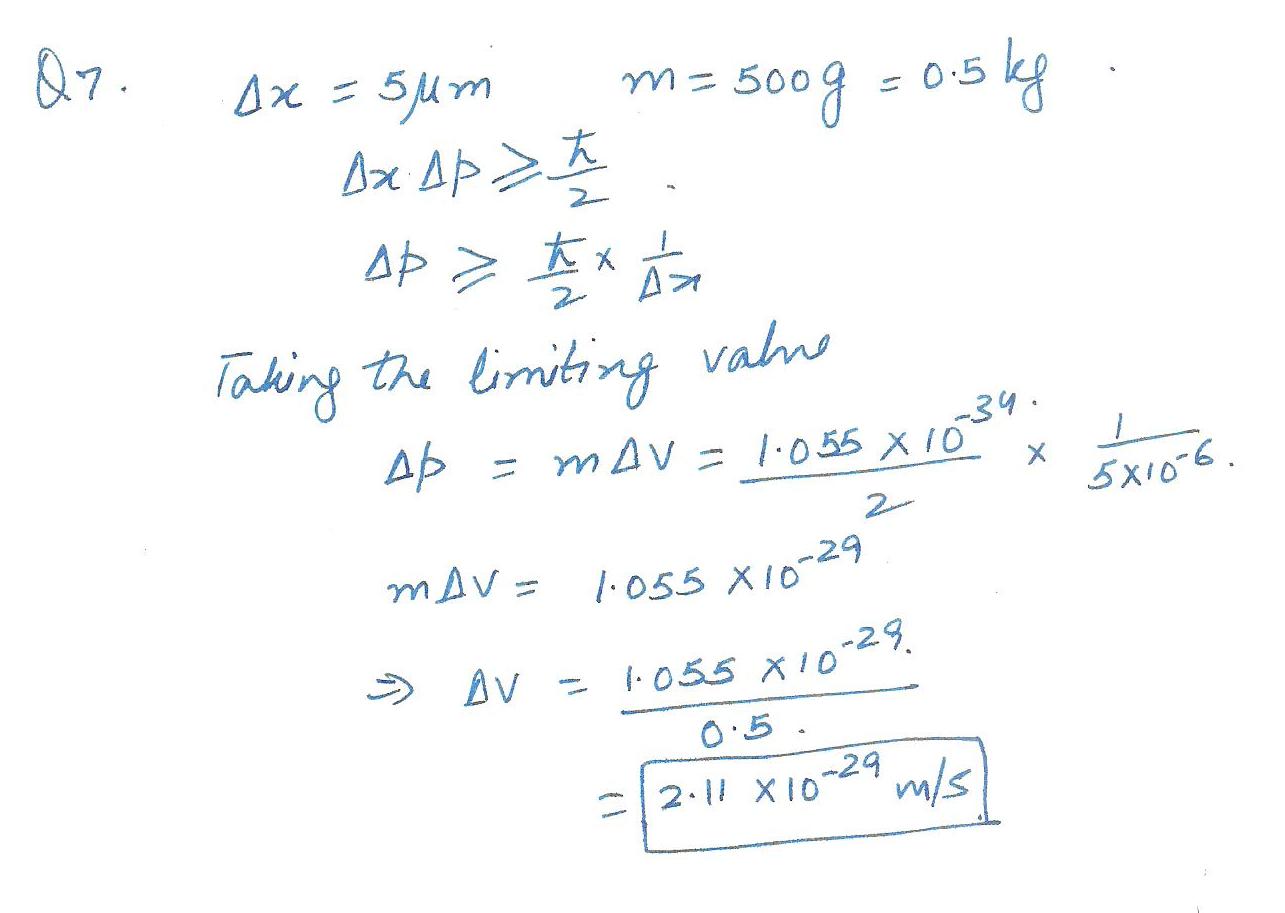
Physics Modern Physics Level: Misc Level
The speed of a certain proton is 350 km/s. If the uncertainty in its momentum is 0.0100 percent, what 7 unceertainty in its location must be tolerated?

Physics Modern Physics Level: Misc Level
The energy required for the ionization of a certain atom is 3.44 aJ (1 aJ==10 to the(-) 18 th J: a denotes atto) The absorption of a photon of unknown wavelength ionizes the atom and ejects an electron with veiocity 1.03x10 to the 6 th m/s. Calculate the wavelength of the incident radiation.
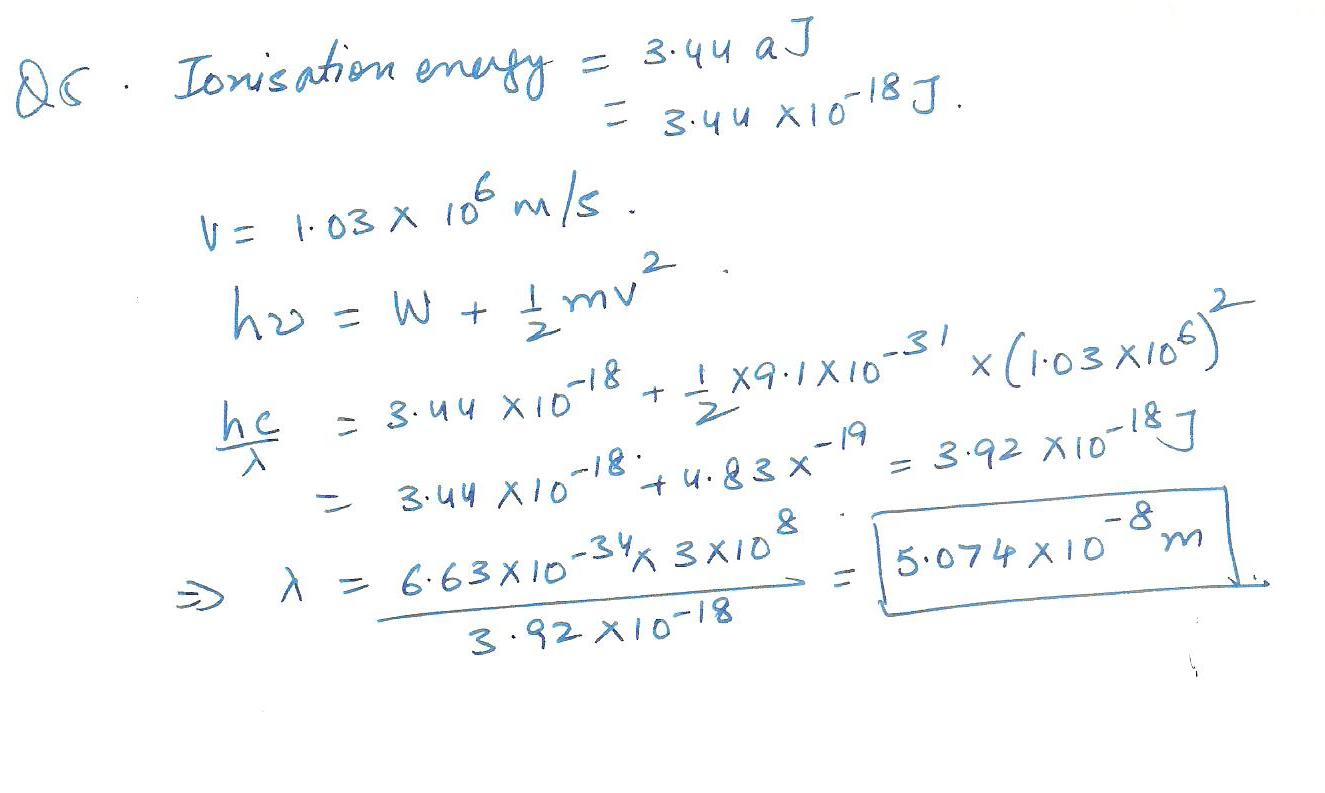
Physics Modern Physics Level: Misc Level
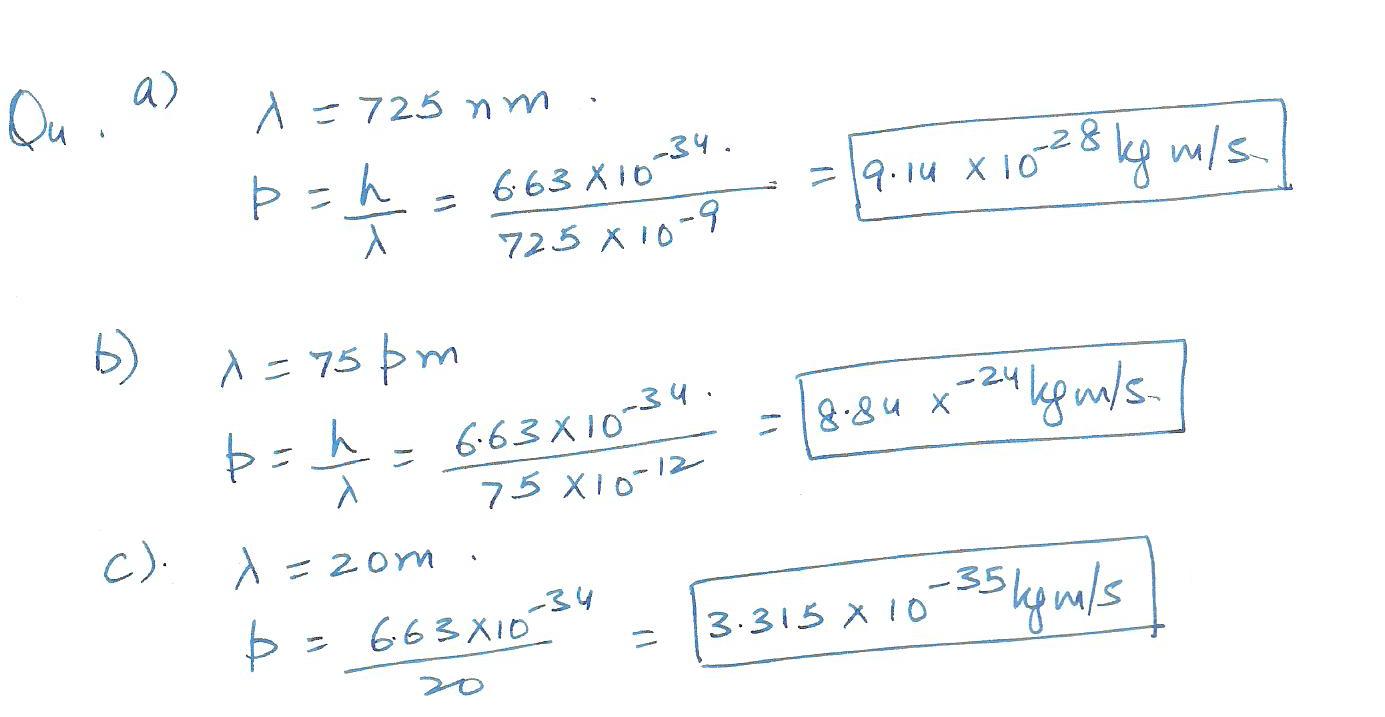
Calculate the linear momentum of photons of wavelength a) 725 nm b)75 pm c) 20 m 2x3 =6.
Physics Modern Physics Level: Misc Level
Calculate the de Broglie wavelength of a) a mass of 1.0 g traveling at 1.0 m/s b) the same traveling at 1.00x10 to the 5 th km/s c)a helium atom traveling at 1000 m/s (a typical speed at room temperature)3x3=9
Physics Modern Physics Level: Misc Level
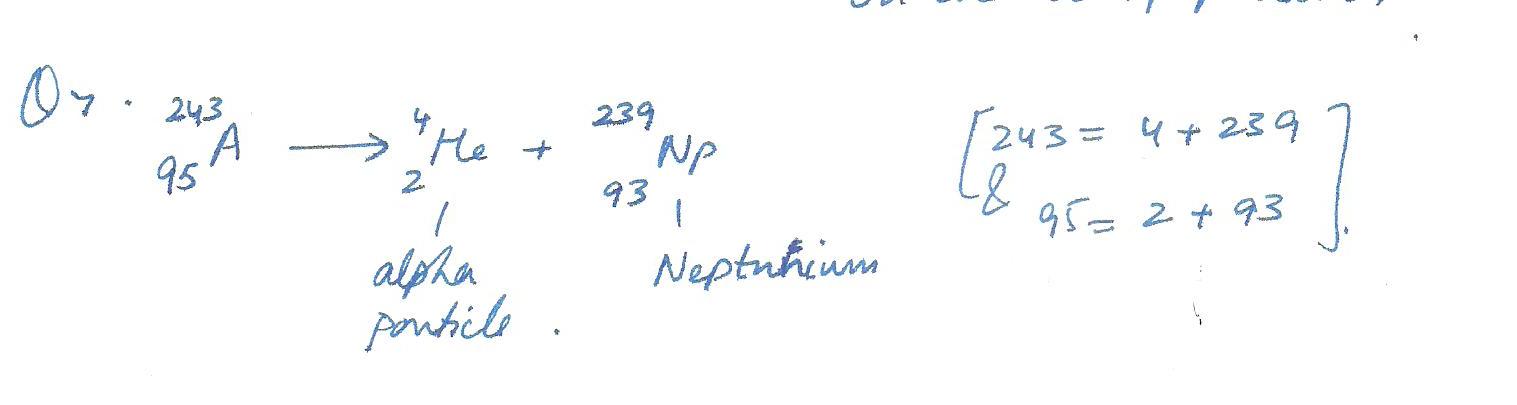
If you have an atom of Americanium (atomic symbol Am) and it undergoes alpha decay, what type of atom will you have after the decay? (If you don,t know its name, just write the correct symbol from the periodic table.)Explain.
Physics Modern Physics Level: Misc Level
Suppose you want to measure the position of an electron to within 1 angstrom. (An angstrom is 1x10-10m).If you also want to measure its momentum, how accurately can you do this if you don't want to mess up your measurement of its position?
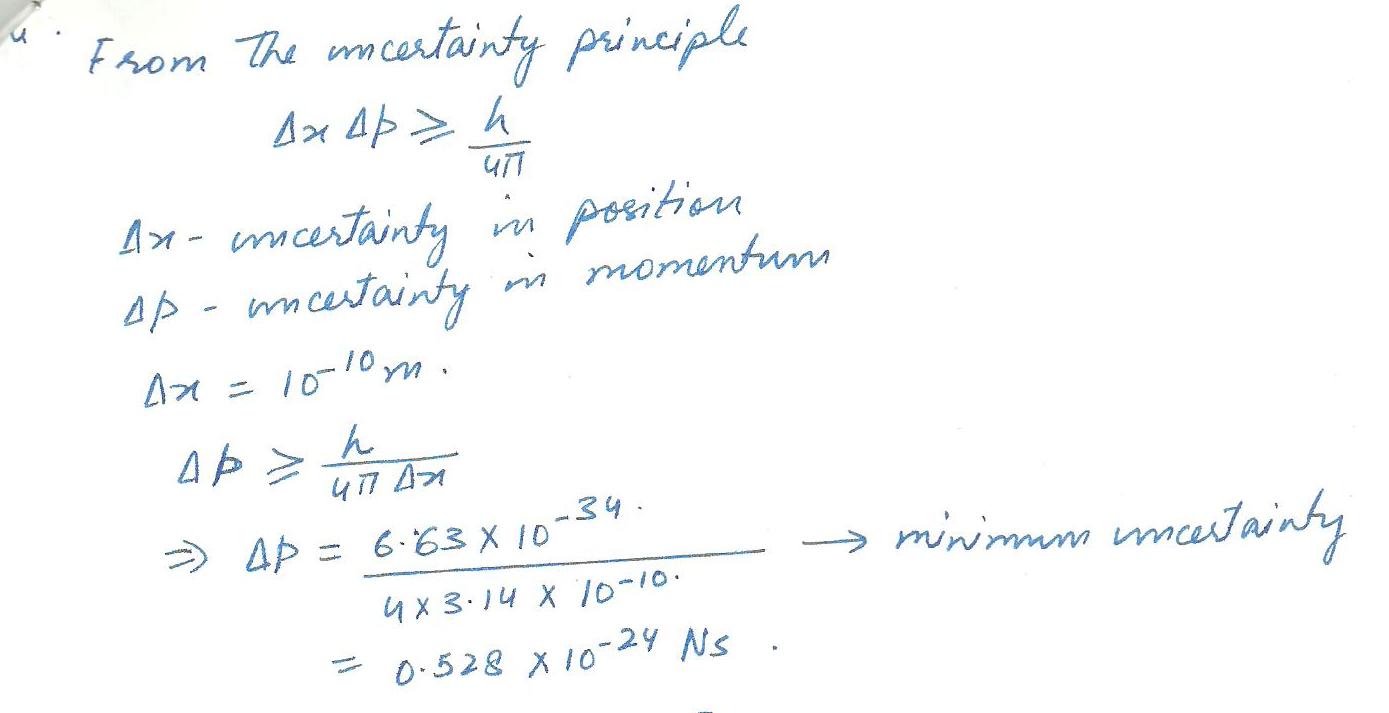
Physics Modern Physics Level: Misc Level
If a photon with (wavelength )=300nm strikes a metal with (angle)=7x10-19J, will any electrons be ejected?Explain.

Physics Modern Physics Level: Misc Level
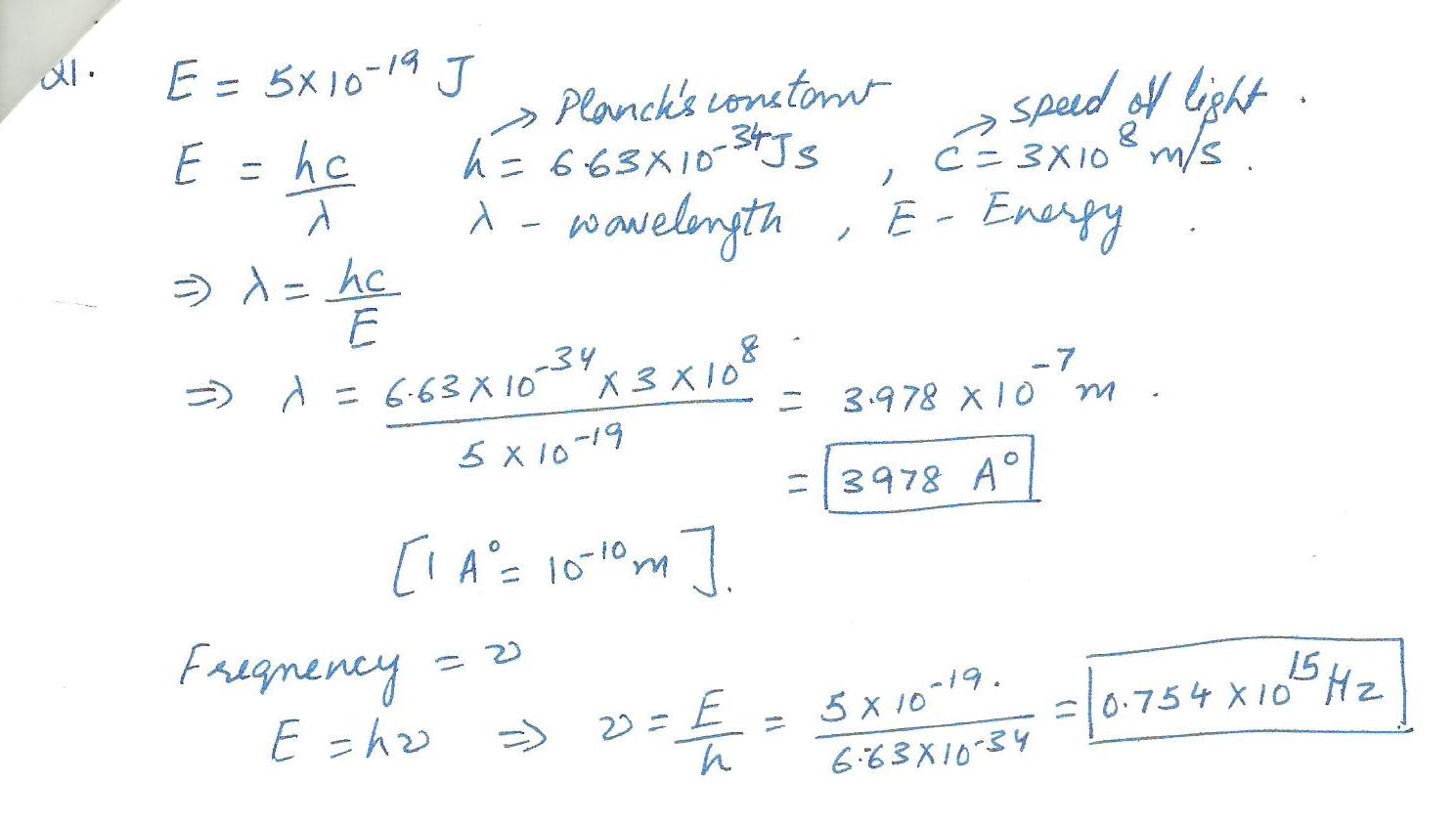
Suppose that a photon has an energy of 5x10-19 J. What,s the wavelength of this photon? What is its frequency?