Physics Properties Of Matter Level: Misc Level
A 70.3 kg person has an apparent mass of 48.7 kg (because of buoyancy) when standing in water that comes up to the hips. Estimate the mass of each leg. Assume the body has SG =1.00
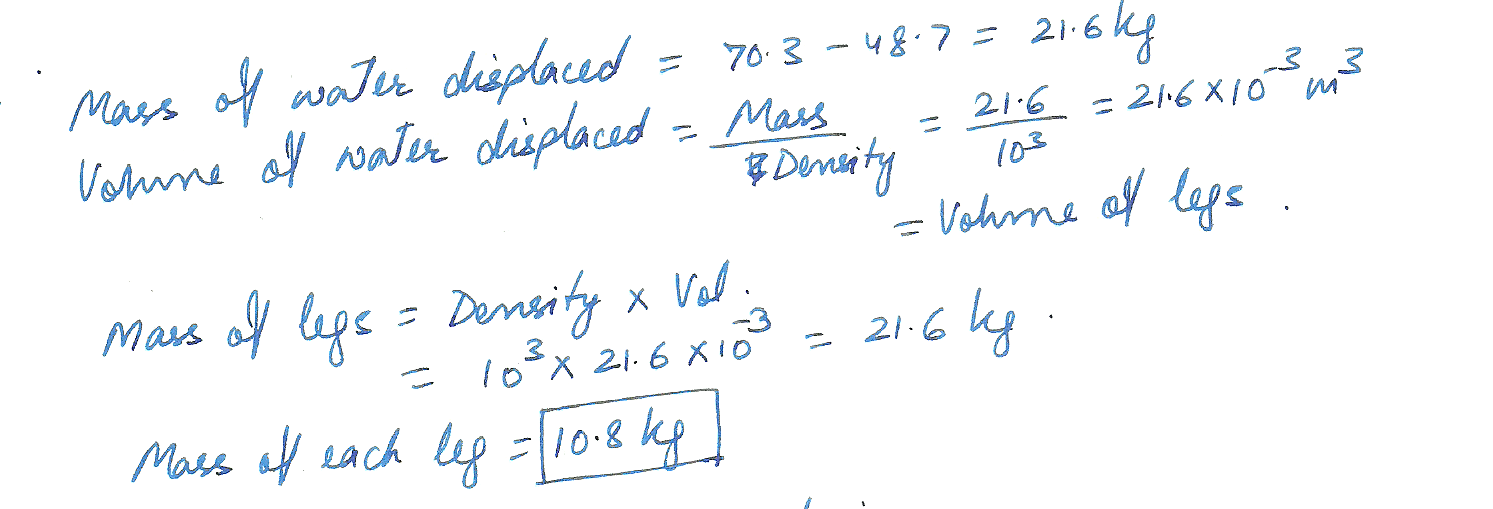
Physics Properties Of Matter Level: Misc Level
A spherically shaped balloon has a radius of 9.54 m, and is filled with helium. How large a cargo can it lift, assuming that the skin and structure of the balloon have a mass of 978 kg? Neglect the buoyant force on the cargo volume itseIf.
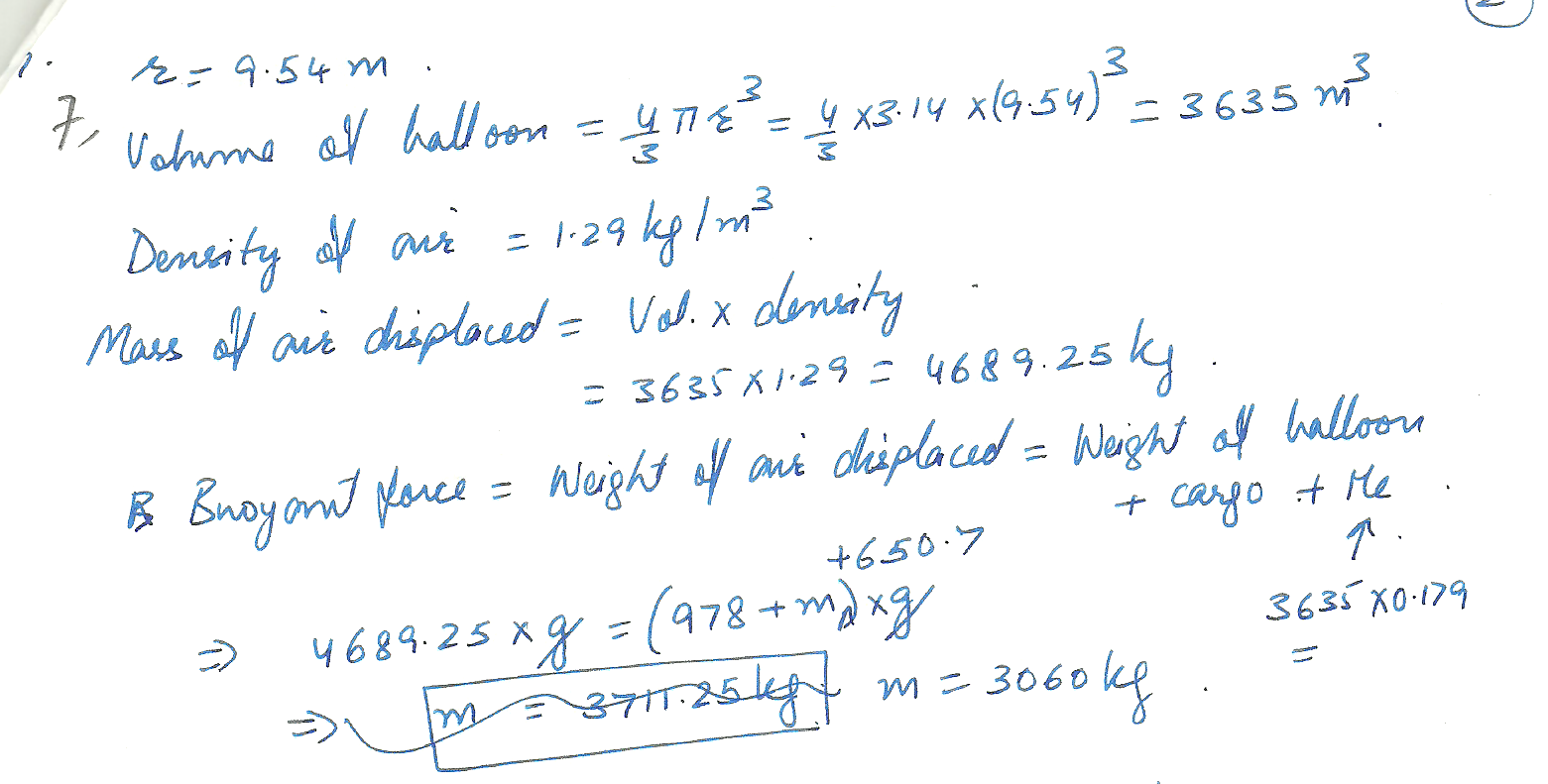
Physics Properties Of Matter Level: Misc Level
Calculate the total force of the atmosphere acting on the top of a table that measures 1.52 m x 2.77 m.
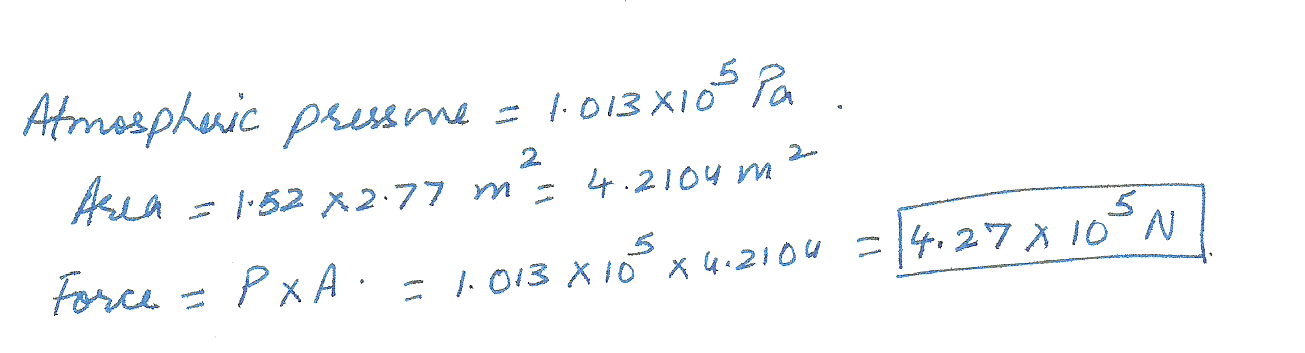
Physics Properties Of Matter Level: Misc Level
If 5.75 liter of antifreeze solution (specific gravity = 0.800) is added to 3.95 liter of water to make a 9. 70 liter mixture, what is the specific gravity of the mixture?Assume the water is at a temperature of 4 degrees C.
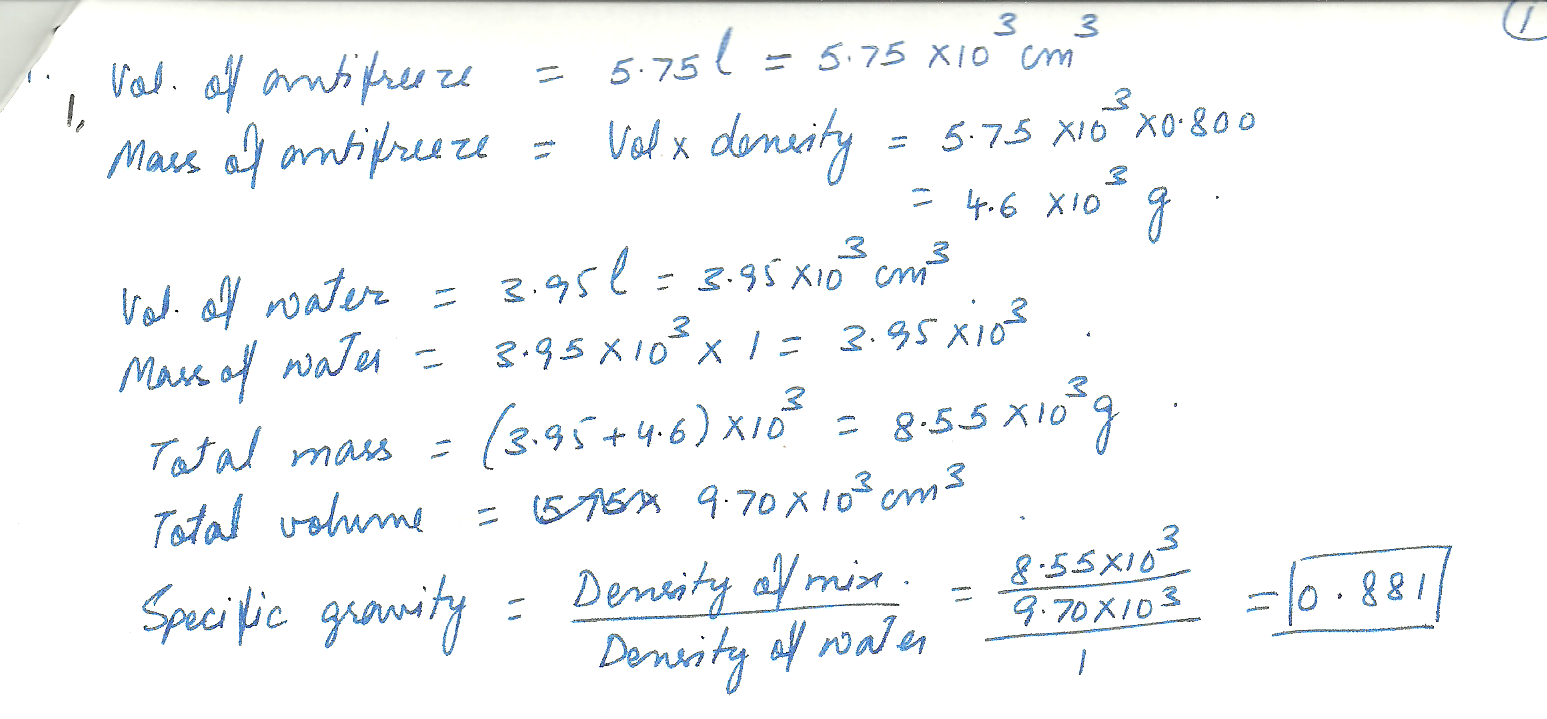
Physics Properties Of Matter Level: Misc Level
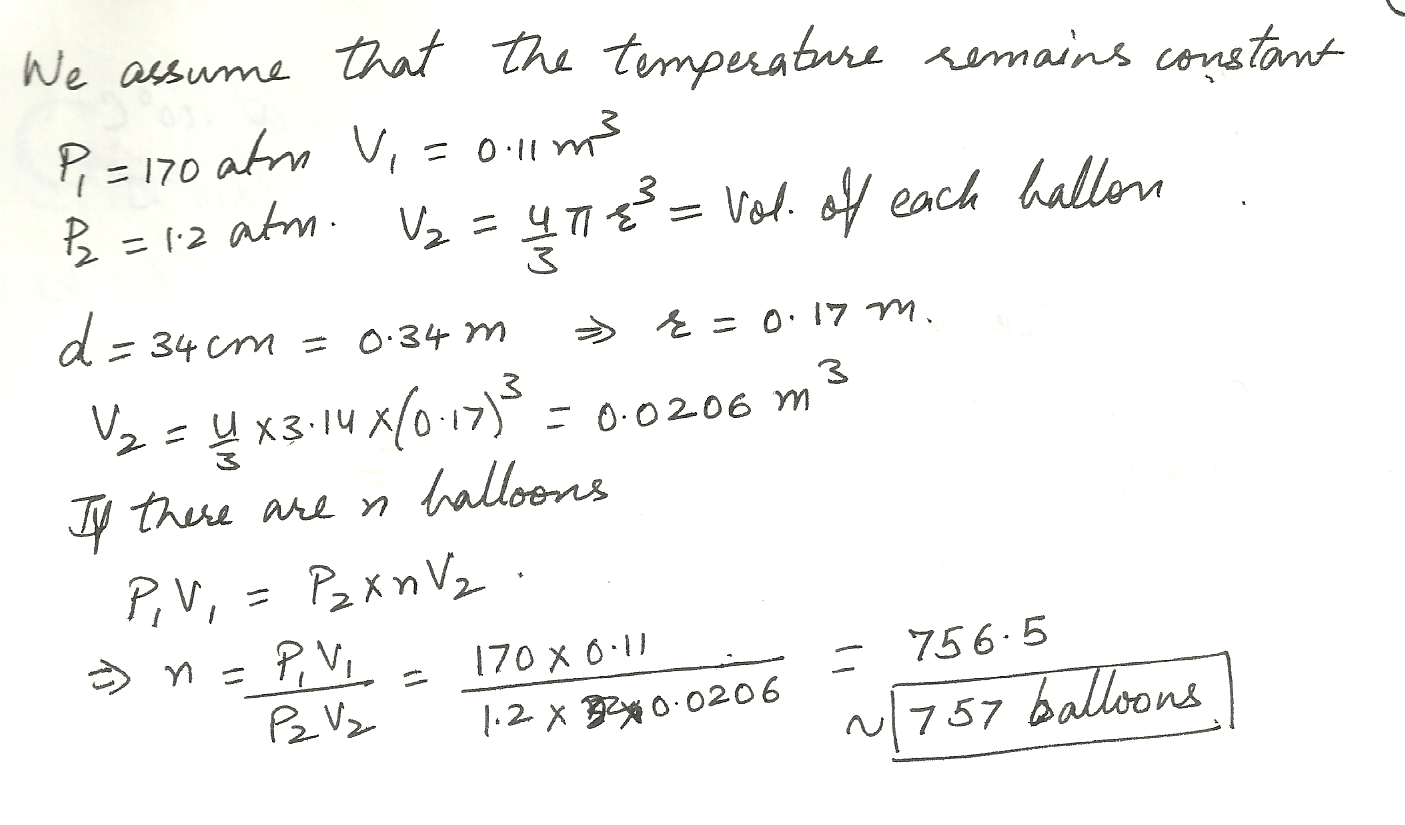
A tank having a volume of 0.11 m3 contains helium gas at 170 atm. How many balloons can the tank completely fill if each filled balloon is a sphere 34 cm in diameter at an absolute pressure of 1.2 atm?
Physics Properties Of Matter Level: Misc Level
A gas cylinder of volume 36 liters contains an ideal gas at temperature 27 % C. and pressure 11720 kPa. Some of the gas leaks, untill the pressure falls to 1325 kPa. How many moles of gas leaked, assuming that the temperature remains constant during this process?

Physics Properties Of Matter Level: Misc Level
A constant volume gas thermometer has a pressure of 7160 Pa at 22 % C. What would the pressure be for - 61 % C. (in Pa)?
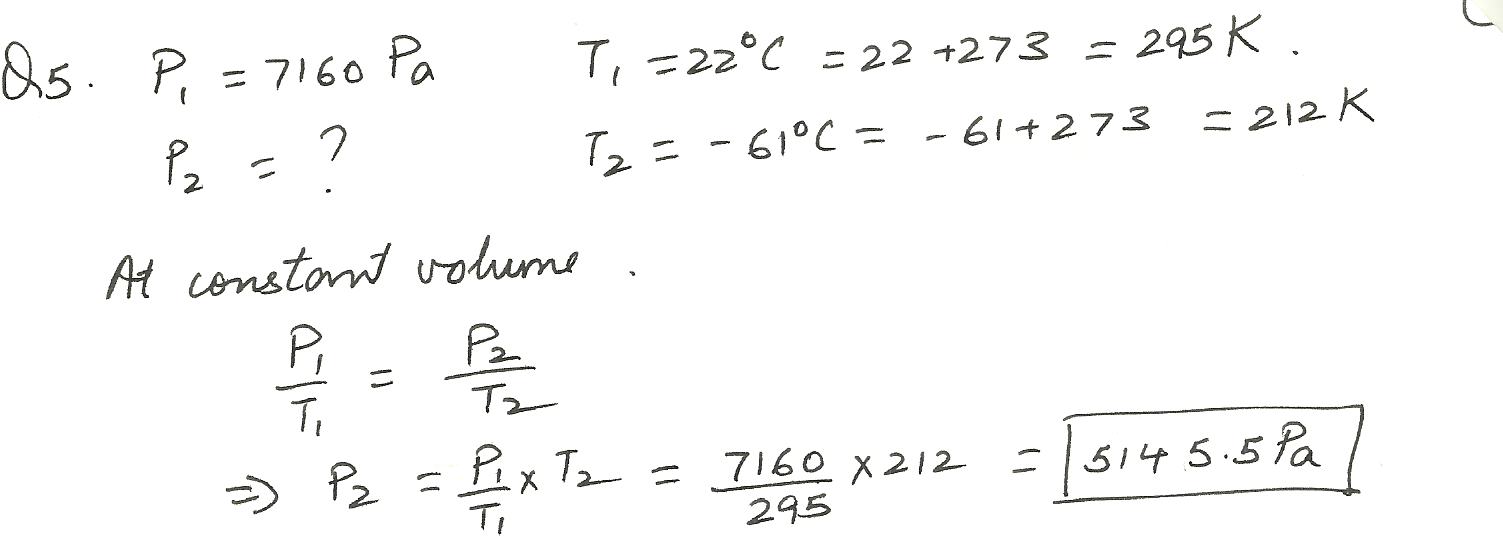
Physics Properties Of Matter Level: Misc Level
A can of gasoline has a rectangular base with dimensions of 13 cm by 10 cm. If there is 0.5 liters of gasoline in the can, how much does the surface of the gasoline rise (in mm) in the can when the temperature is raised by 34%C.
The coefficient for volume expansion of gasoline is 9.5 10-4 /%C.
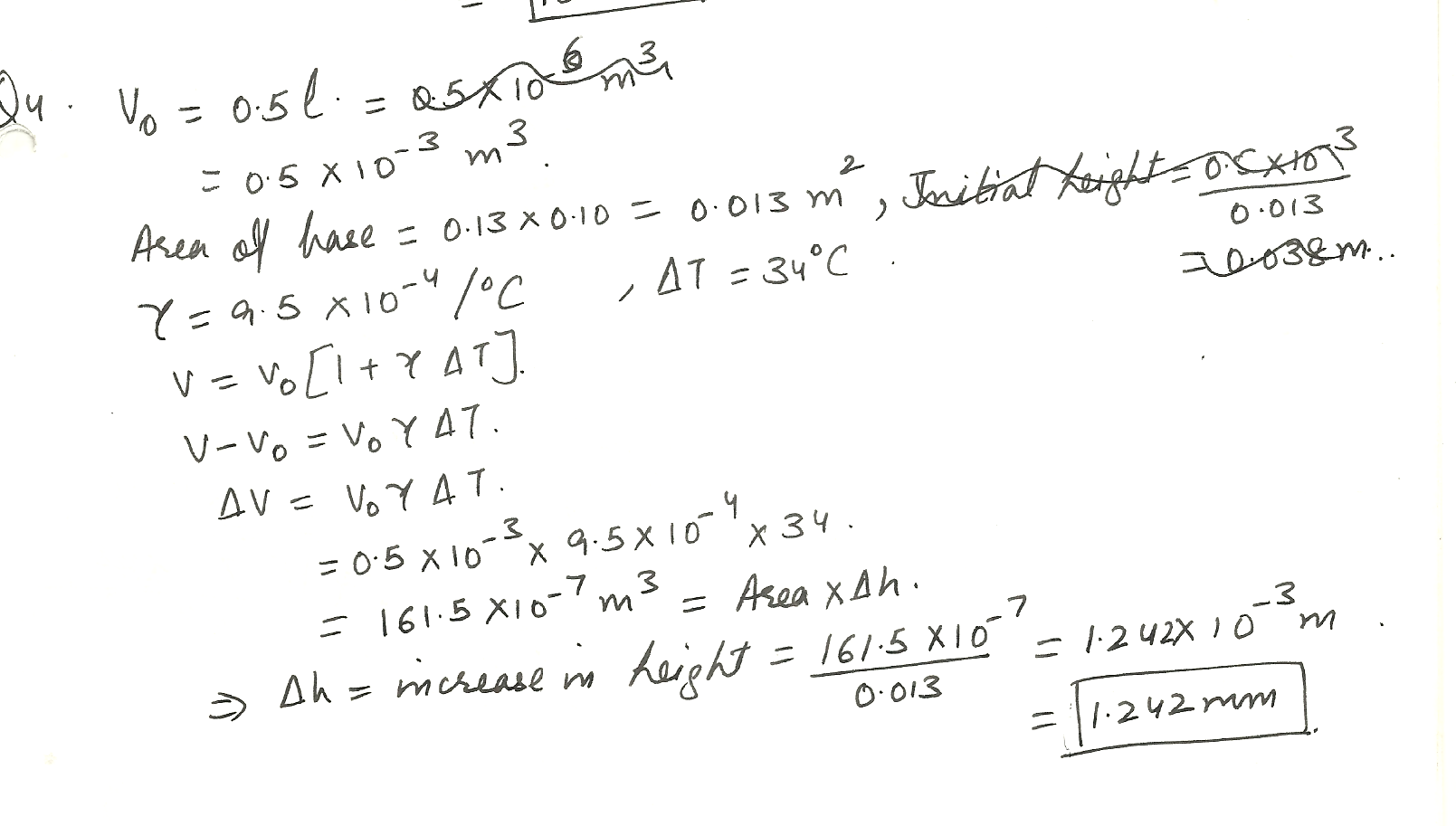
Physics Properties Of Matter Level: Misc Level
2.9 liters of Nitrogen at - 2 %C and 2.3 atmospheres contain how many moles?
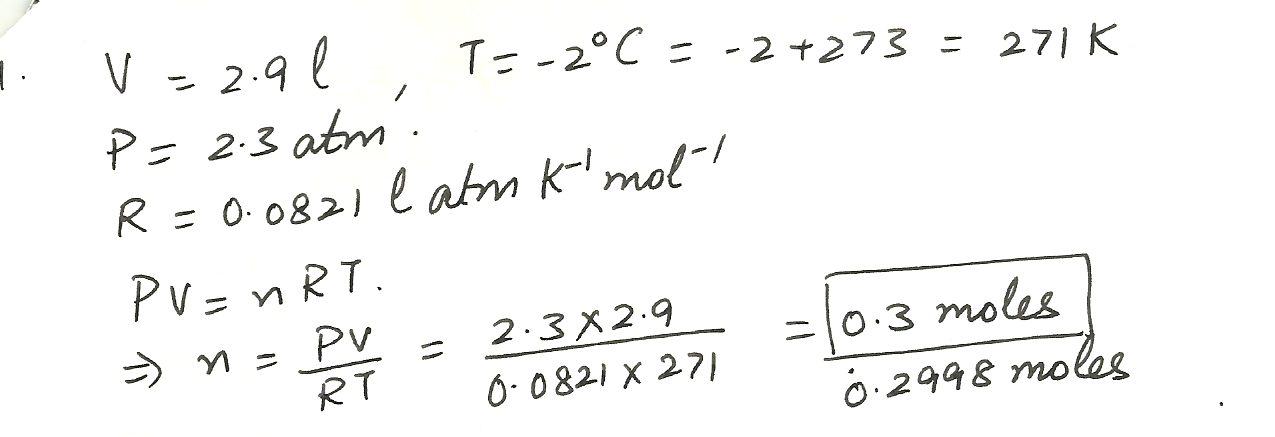
Physics Properties Of Matter Level: Misc Level
A collapsible plastic bag contains a glucose solution. If the average gauge pressure in the vein is 1. 33 x 10 ^3 Pa, what must the minimum height of the bag in order to infuse glucose into the vein? Assume the specific gravity of the solution is 1. 02.
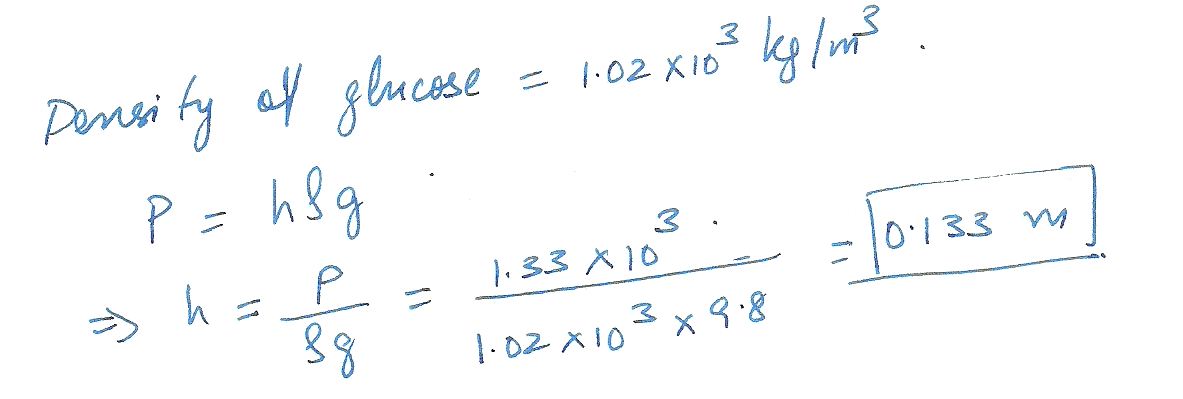
Physics Properties Of Matter Level: Misc Level
If the elastic limit of steel is 5.0 x 10 ^ 8 Pa, determine the minimum diameter a steel wire can have if it is to support a 70 - kg circus performer without its elastic limit being exceeded.
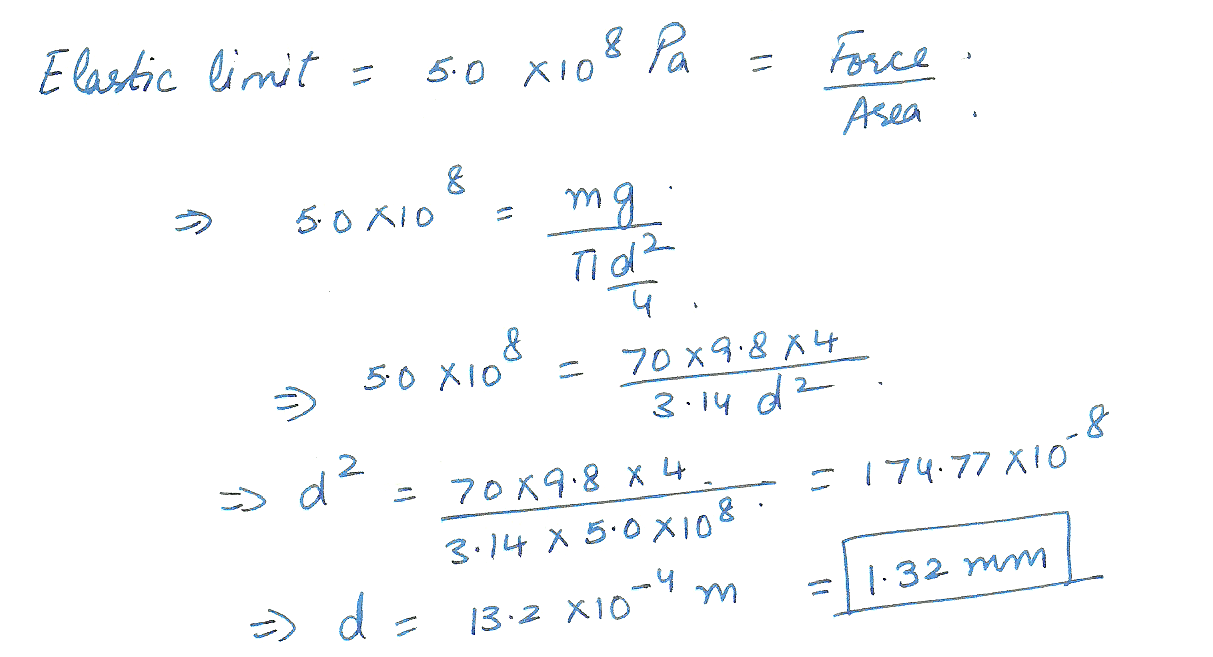
Physics Properties Of Matter Level: Misc Level
A block of ice at 0% is added to a 150 g aluminum calorimeter cup that holds 200 g of water at 10% C If all but 2.00 g of ice melt, what was the original mass of the block of ice?
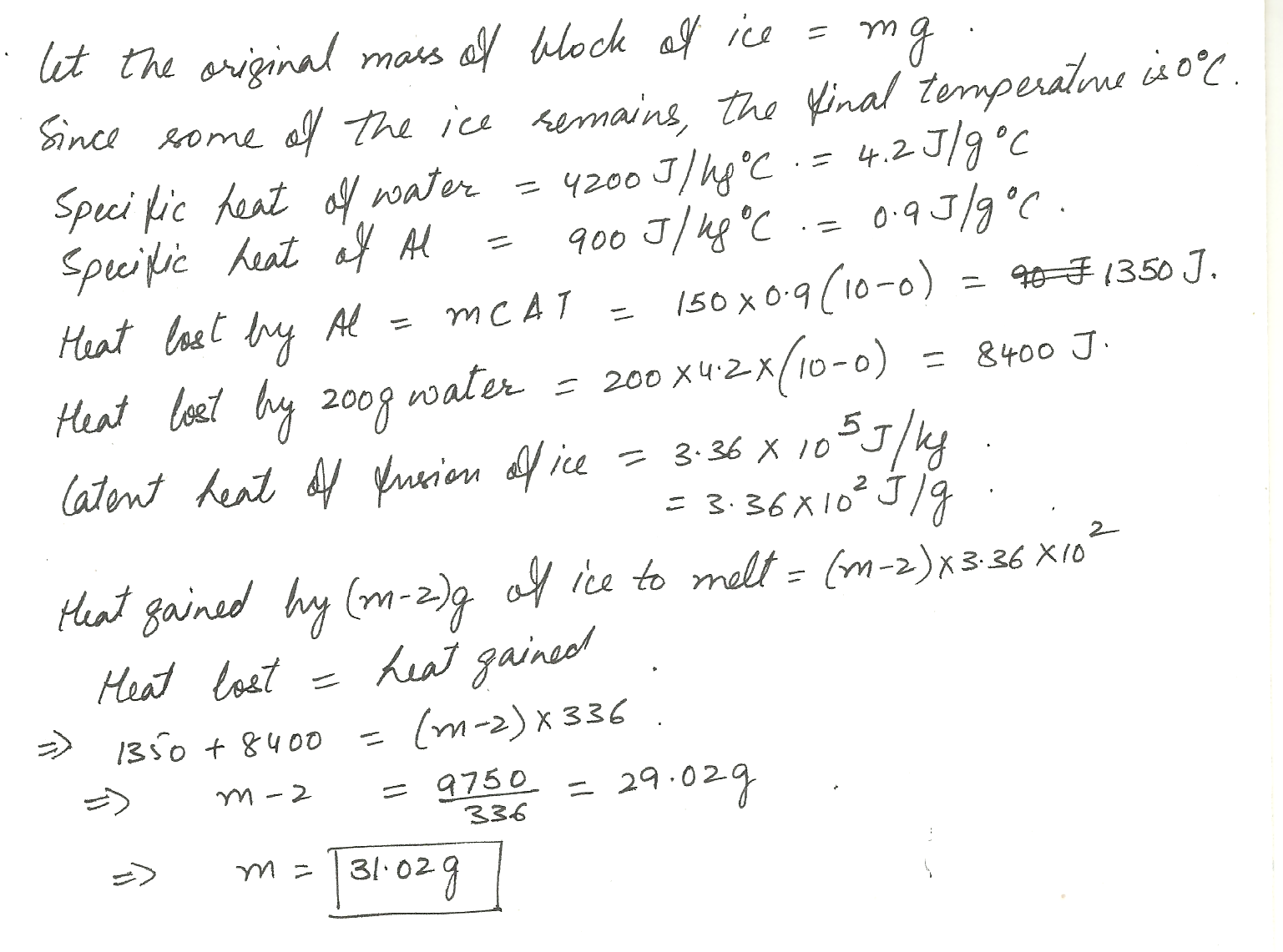
Physics Properties Of Matter Level: Misc Level
If the density of gold is 19.3 x103 kg/m3 what buoyant force does a 0.60 kg gold crown experience when it is immersed in water?
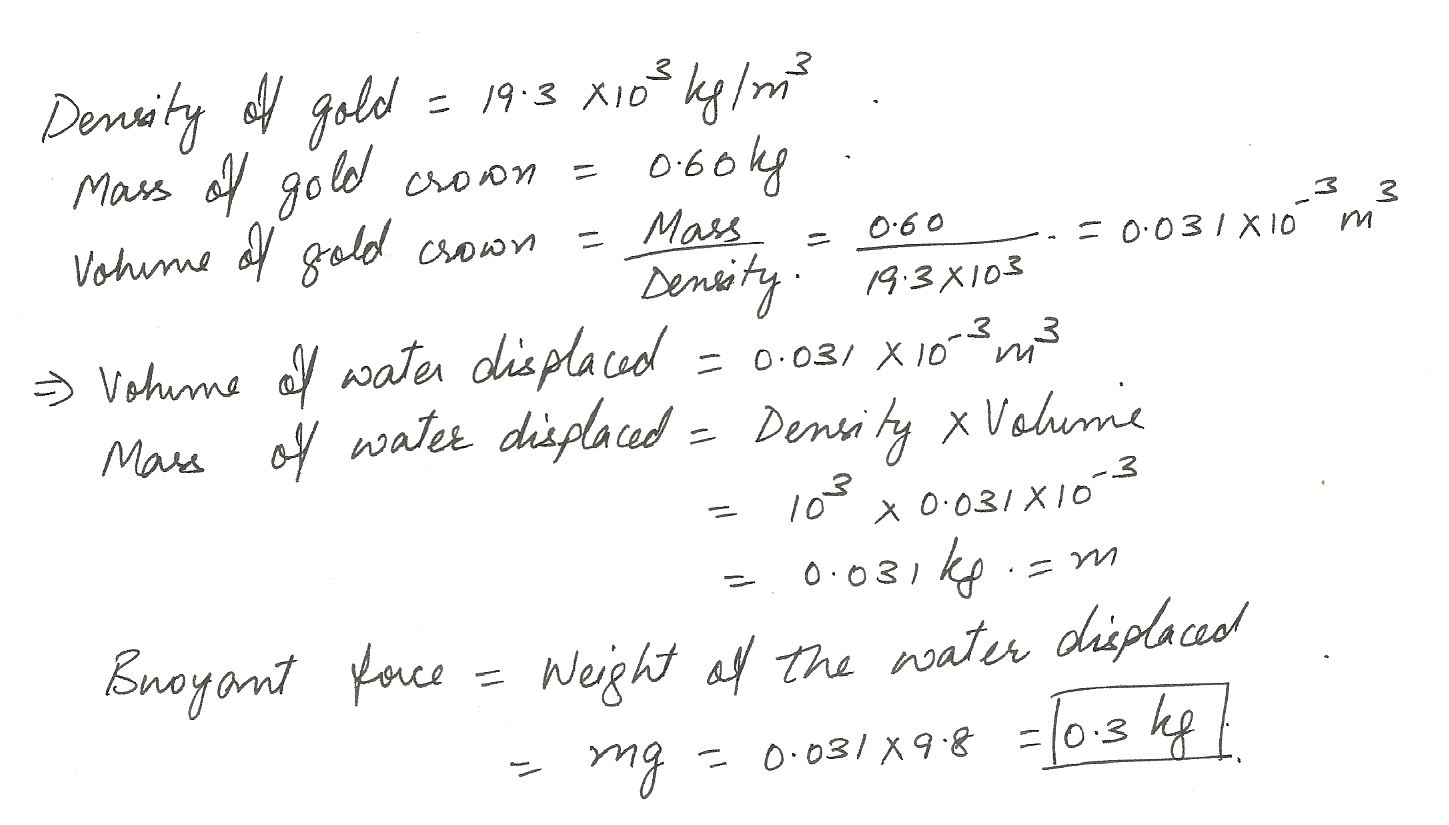
Physics Properties Of Matter Level: Misc Level
Water is pumped through a pipe of diameter 15.0 cm from the Colorado River up to Grand Canyon Village, on the rim of the canyon. The river is at 564 m elevation and the village is at 2096 m. (a)At what minimum pressure must the water be pumped to arrive at the village? (b) If 4500 m^3 are pumped per day, what is the speed of the water in the pipe? (c) What additional pressure is necessary to deliver the flow? (note You may assume that the freefall acceleration and the density of the air are constant over the given range of elevations.)

Physics Properties Of Matter Level: Misc Level
Air is trapped above liquid ethyl alcohol in a rigid container. If the air pressure above the liquid is 1.10 atm, determine the pressure inside a bubble 4.0 m below the surface of the liquid.

