Physics Circular Motion Level: Misc Level
An angular acceleration of 1200 rev/min2 when expressd in radians2 is:
a. 20 rad/s2
b. 1.2 rad/s2
c. 2.1 rad/s2
d. 0.20 rad/s2
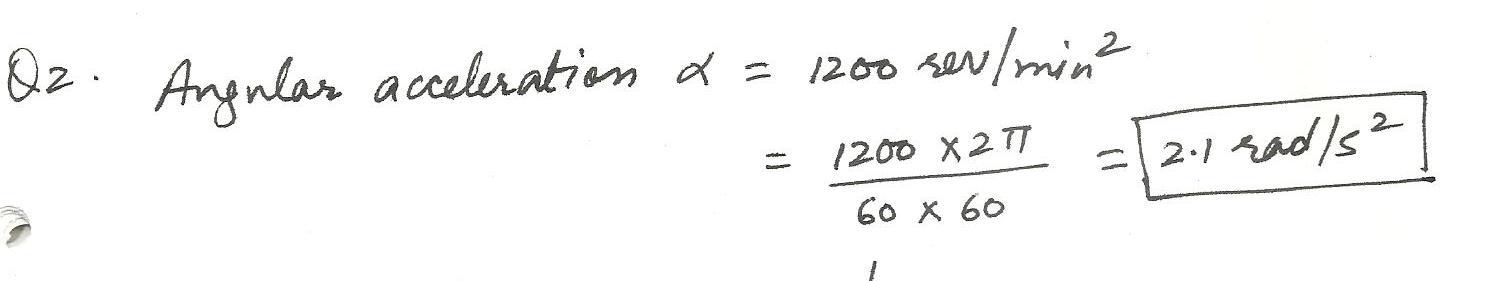
Physics Force & Laws Of Motion Level: Misc Level
An object with a mass of 2 kg traveling with a velocity of 3 m/s, collides- on and elastically with a stationary object of mass 0.5 kg. The velocity of the 2 kg mass after the collision is:
a. 1 m/s
b. 1.5 m/s
c. 2.3 m/s
d. 1.8 m/s

Chemistry Inorganic Chemistry Level: Misc Level
According to VSEPR theory, which of the following shapes is possible for a molecule with he molecular of AB2?
a. linear
b. bent
c.trigonal planar
d. both(a)&(b)
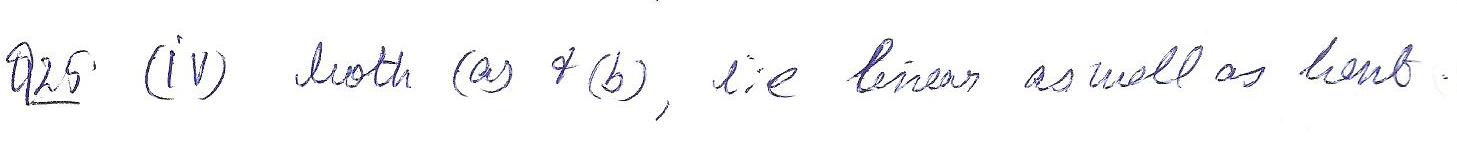
Chemistry Inorganic Chemistry Level: Misc Level
Name the following compound (NH4)3PO4
a.Ammonium Phosphite
b. Tri-ammonium Phospho tetraoxide
c. Ammonium Tri-phosphide
d.Ammonium Phosphate

Chemistry Inorganic Chemistry Level: Misc Level
Name the following compound (NH4)3PO4
a.Ammonium Phosphite
b. Tri-ammonium Phospho tetraoxide
c. Ammonium Tri-phosphide
d.Ammonium Phosphate

Chemistry Inorganic Chemistry Level: Misc Level
Which of the following molecules would most likely be classified as a tetrahedral shape?
a. HCI
b. CO
c. C4H8
d. CH4
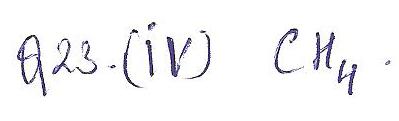
Chemistry Inorganic Chemistry Level: Misc Level
To draw a Lewis structure, you DO NOT need to know
a. the number of valence electrons for each atom
b. the types of atoms in the molecule
c. the number of atoms in the molecule
d. bond energies

Chemistry Inorganic Chemistry Level: Misc Level
One can predict the shape of a molecule by drawing its Lewis structure and
a. balancing the oxidatin numbers
b. accounting for nonbonded pairs of valence electrons
c. determing the empirical formula
d. identifying the presence of polyatomic ions
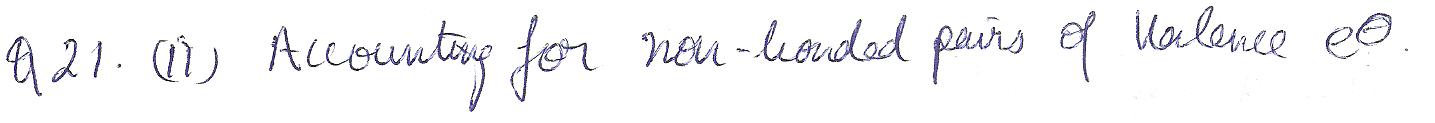
Chemistry Inorganic Chemistry Level: Misc Level
According to the VSEPR theory the molecular shape of CO2, carbon dioxide,is classified as
a.linear
b.bent
c. trigonal planar
d. trigonal pyramidal
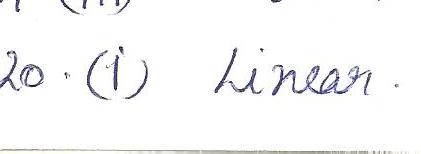
Chemistry Inorganic Chemistry Level: Misc Level
The two suffixes that indicate the presence of oxygen in a polyatomic ion are
a.-ite &- ide
b. - ite & -ide
c. -ate &- ide
d.- thio &- penta
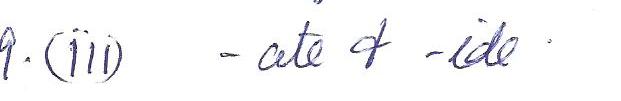
Chemistry Inorganic Chemistry Level: Misc Level
The lewis structure for methane, CH4, has
a. 2 double bonds
b. 1 triple bond
c. 4 single bonds
d. 2 single bonds & 2 double bonds

Chemistry Inorganic Chemistry Level: Misc Level
the correct formula for Magnesium Oxide is
a.MgO2
b.MgO
c Mg2O
d.Mg2O3

Chemistry Inorganic Chemistry Level: Misc Level
VSEPR theory staes that the repulsion between electron pairs surrounding an atom causes
a.these pairs to be as far apart as possible
b.an electron sea to form
c.a bond to break
d.a covalent bond to form

Chemistry Inorganic Chemistry Level: Misc Level
the charge of an ion is
a. always positive
b. always negative
c.either positive or negative
d.zero

Chemistry Inorganic Chemistry Level: Misc Level
The tendency of atoms to gain or lose electrons so that their ouer orbitals are filled with electrons is the
a.vseper theory
b. Quartet rule
c.Octet Rule
dElectronegativity rule

