Physics Quantum Mechanics Level: High School
(a) Find the expectation value (b) Find the probability that the electron is at a distance greater that
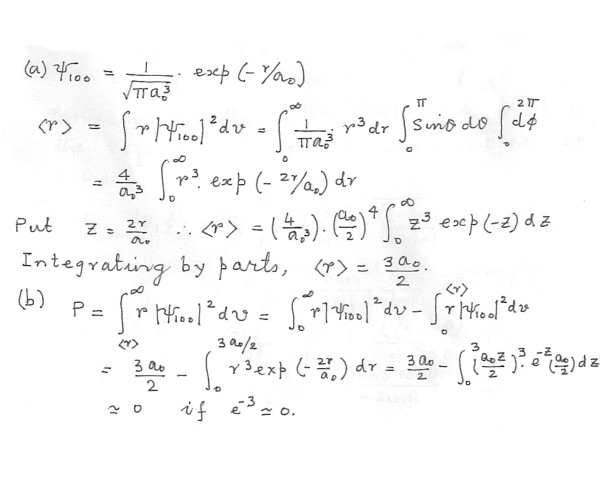
Physics Quantum Mechanics Level: High School
Compare the wave lengths of the 2P„³ 1S transition in (1) hydrogen . (1) deuterium (nuclear mass = 2 x proton mass) , (3) positronium (a bound state of an electron and a positron , whose mass is the same as that of an electron ) .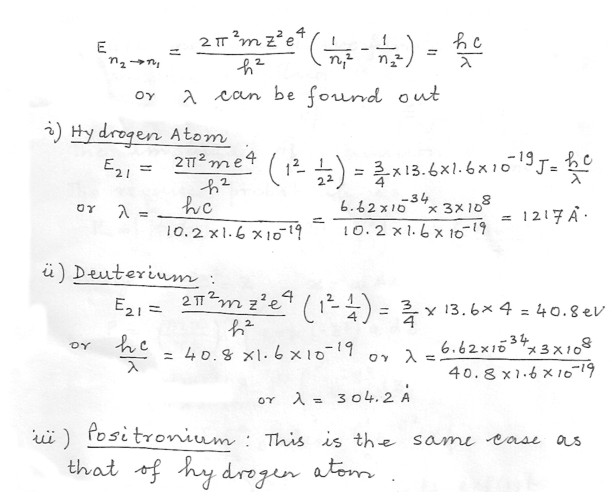
Physics Quantum Mechanics Level: High School
An electron in the coulomb field of a proton is in a state described by the wave function 1/6[4ø100(r) + 3ø211(r)-ø210(R) + (10) ½ ø21-1 (R)](a) What is the expectation value of the energy ?
(b) What is the expectation value of L2 ?
(c) What is the expectation value of Lz ?

Physics Relativity Level: High School
The K^0 particle decays according to the equation K^0->pi++pi- . If a particular K^0 decays while it is at rest in a laboratory , What are the kinetic energies of each of two points ? (The rest mass of the K^0 is 497.7 MeV/c^2)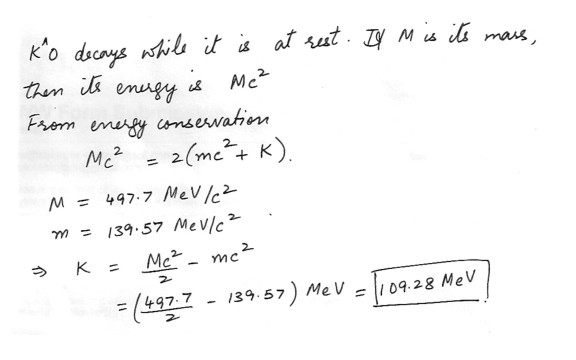
Physics Heat & Thermodynamics Level: High School
A 57.1 kg ice –skater moving at 6.44 m/s glides to a stop. Assuming the ice is at 0 degreeC and that 59.2 percent of the heat generated by friction is absorbed by the ice , how much ice melts ?
Physics Heat & Thermodynamics Level: High School
A hot iron horseshoe (mass = 0.440 kg ) which has just been forged , is dropped into 1.76 L of water in a 0.200 kg iron pot initially at 20.4 degree C , if the final equilibrium temperature is 25.6 degreeC , calculate the initial temperature of the hot horseshoe ( in Celsius )
Physics Heat & Thermodynamics Level: High School
At a crime scene, the forensic investigator notes that 11.3 –g lead bullet that was stopped in a door frame apparently melted completely on impact. Assuming the bullet was fired at room temperature of 20 degreeC , What does the investigator calculate the minimum muzzle velocity of gun was ?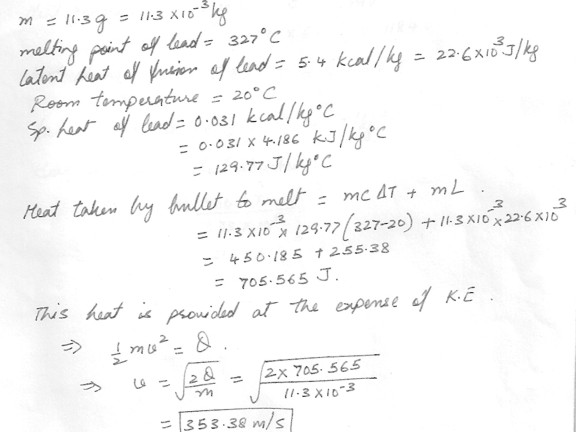
Physics Heat & Thermodynamics Level: High School
A brass plug is to be placed in a ring made of iron. At room temperature , the diameter of the plug is 8.791 cm and that of the inside of the ring is 8.786cm. They must both be brought to what common temperature (in degreeC) in order to fit ?
Physics Heat & Thermodynamics Level: High School
The temperature of an ideal gas is increased from 136 degreeC to 368 degreeC while the volume and the number of mole stay constant . By what factor does the pressure change ?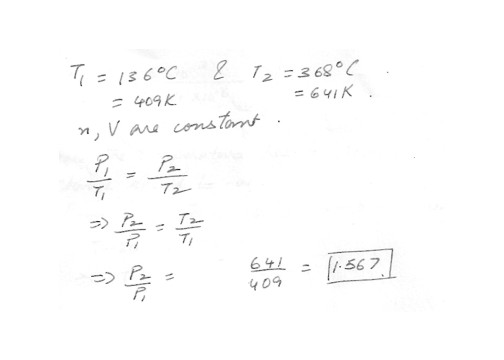
Physics Heat & Thermodynamics Level: High School
To make a secure fit , rivets that are larger than the rivet hole are often used and the rivet is cooled (usually in dry ice) before it is placed in the hole . A steel rivet 1.818 cm in diameter is to be placed in a hole 1.814 cm in diameter . To what temperature (on the Celsius scale) must the rivet be cooled if it is to fit in the hole (at 20.0 degreeC) ?
Physics Heat & Thermodynamics Level: High School
0.1 kg of water at 92.0 degree C is poured into an insulated cup containing 0.246 kg of ice initially at 0 degreeC . Calculate the mass of liquid when the system reaches thermal equilibrium .
Physics Heat & Thermodynamics Level: High School
(a) If 0.8 mol of a gas in a container occupies a volume of 14.01 at a temperature of 1.2 atm, What is the temperature of the gas (in K) ?(b) The container is fitted with piston so that the volume can change . When the gas is heated at constant pressure , it expands to a volume of 19.01 What is the temperature of the gas (in K) ?
(c) The volume is fixed at 19.01 and the gas is heated at constant volume until its temperature is 333.0 K . What is the pressure (in atm) of the gas now ?
Physics Heat & Thermodynamics Level: High School
(a) Lake Erie contains roughly 3.90 E + 11 m^3 of water. How much heat is required to raise the temperature of that volume of water from 10.8 degreeC to 13.0 degreeC ?(b) How many years would it take to supply this amount of heat by using the full output of a 1150 MW electric power plant ? Do not enter unit

Physics Heat & Thermodynamics Level: High School
A class of 10 students taking an exam has a power output per student of 124 W. Assume that the initial temperature of the room is 18.0 degreeC and that its dimensions are 5.90 m by 14.5 by 3.00m . What is the temperature (in degree C , do not enter units) of the room at the end of 61.0 min if all the heat remains in the air in the room and none is added by an outside source ? The specific heat of air is 834 J/kg*degreeC , and its density is about 1.32 E-3g/cm^-3 .
Physics Heat & Thermodynamics Level: High School
A 110 g ice cube at 0 degreeC is placed in 628 g of water at 25.2 degreeC . What is the final temperature of the mixture ? Do not enter units .
