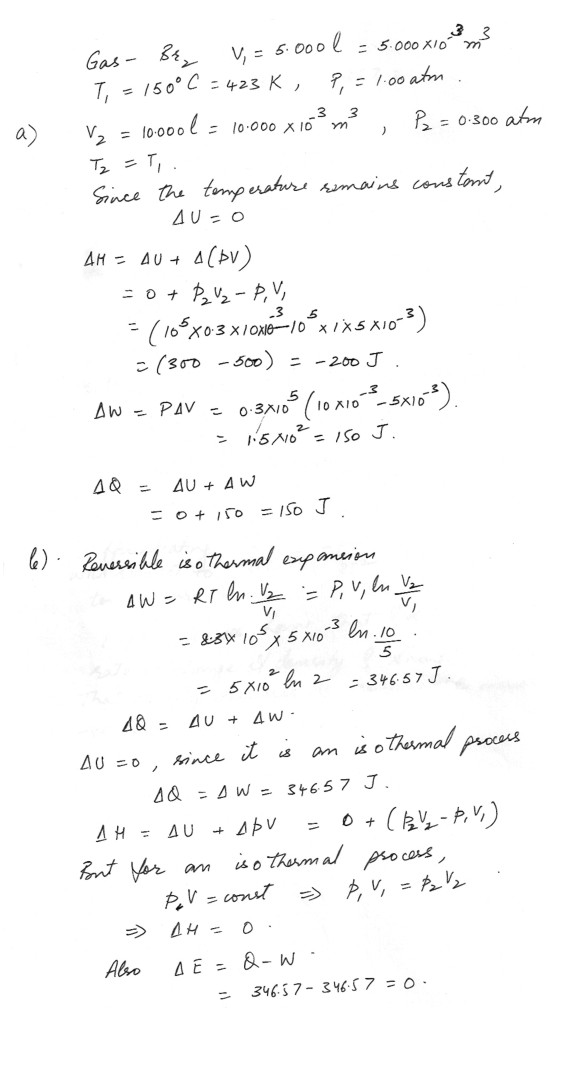Physics Heat & Thermodynamics Level: High School
How much heat is required to change a 46.8 g ice cube from ice at -10.8 degreeC to steam at 115 degreeC ?
Physics Heat & Thermodynamics Level: High School
A 71.4 kg weight-watcher wishes to climb a mountain to work off the equivalent of large piece of chocolate cake rated at 470 (food) Calories . How high must the person climb ? ( 1 food calorie = 10^3 calories)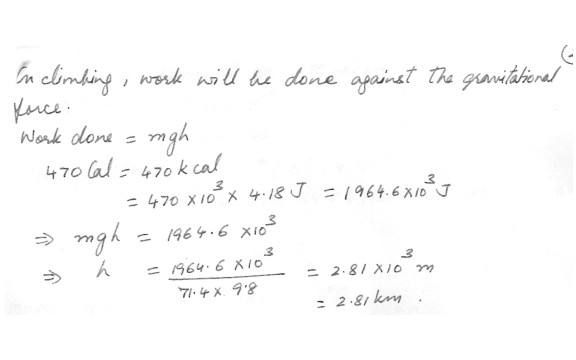
Physics Heat & Thermodynamics Level: High School
An air bubble has a volume of 1.49 cm^3 when it is released by a submarine 104 m below the surface of a lake . What is the volume of the bubble when it reaches the surface ? Assume that the temperature of the air in the bubble remains constant during ascent ?
Physics Heat & Thermodynamics Level: High School
Gas is confined in a tank at a pressure of 11.8 atm and a temperature of 14.1 degreeC . If half of the gas is withdrawn and the temperature is raised to 69.8 degreeC , What is the new pressure in the tank ?
Physics Heat & Thermodynamics Level: High School
You picked up an iron shot-put ball a track meet and were surprised at how heavy it was , it had a mass of 7.25 kg . The iron had the same temperature as the atmosphere (25 celsius) . How many calories must the iron ball absorb to reach its melting point and melt ?(specific heat of iron = 0.11 cal/g Celsius, melting point of iron = 1530 celsius , heat of fusion of iron = 22.0 cal/g)

Physics Heat & Thermodynamics Level: High School
How many kg of Al is experience the same temperature rise as 3 kg of Cu when the same amount of heat is added to each one ?[Clue to solve : 0.05 kg Al @ 20 degree C . Add 200J of heat . What is the final temperature ]
Physics Heat & Thermodynamics Level: High School
8 meters long steel rails (coefficient of linear equation = 12 x 10^-6) are laid end to end in winter when the temperature is -10 degree C . How much space should be left between them to allow for expansion in the summer , when the temperature could reach 50 degree C ? 64.5 degree C ?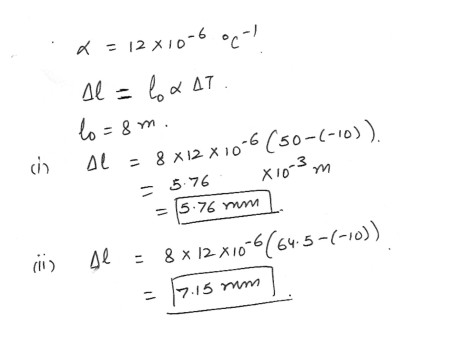
Physics Ray Optics Level: High School
Yellow bright light travels through a glass block at 1.95 x 10^11 m/s . Its wavelength in the glass is 3.81 x 10^-7 m . What is the frequency of the yellow bright light ?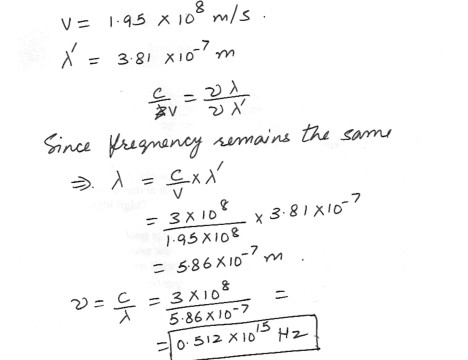
Physics Heat & Thermodynamics Level: High School
Calorimetry
An aluminum calorie meter of mass 50 g contains 95 g of a mixture of water and ice at 0 degrees C . When 100 g of aluminum at 100 degreesC is dropped into the mixture , the temperature rises to 5 degree C . The specific heat of aluminum is 0.22 cal/g - “C . Find the mass of the ice originally present .
Physics Heat & Thermodynamics Level: High School
Calorimetry
An ideal gas is contained within a volume of 2 ft^3 when the pressure is 137 atmospheres and the temperature is 27 degreeC . What volume would this gas occupy if it were allowed to expand to atmosphere pressure at a temperature of 50 degree C .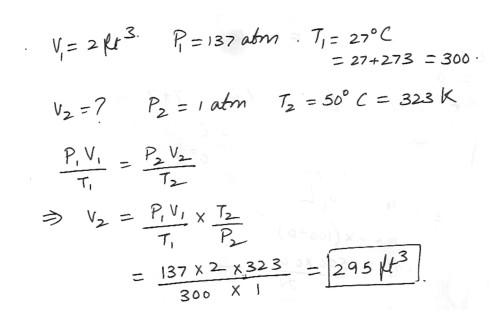
Physics Heat & Thermodynamics Level: High School
Calorimetry
When 2 pounds of brass at 212 degrees F is dropped into 5 lbs of water at 35 degrees F , the resulting temperature is 41.2 degrees F . Ignore the effect of the container . Find the specific heat of the brass .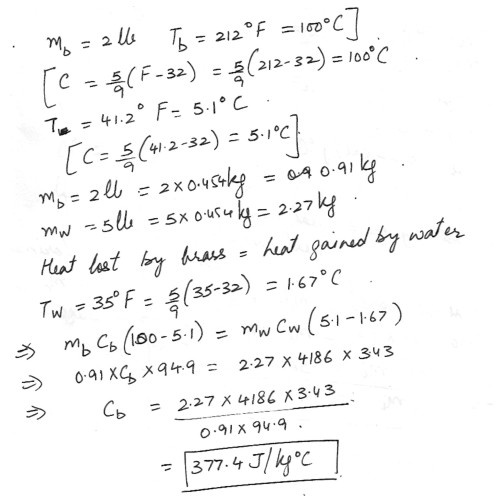
Physics Heat & Thermodynamics Level: High School
Calorimetry
500 g of lead at a temperature of 100 degreeC is poured into a hole in a large block of ice . The specific heat of lead is –“C 0.03 cal/g-“ . How much ice is melted ?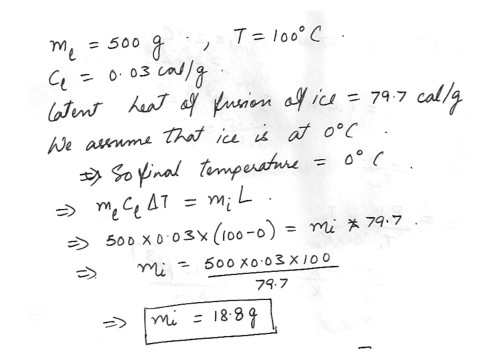
Physics Heat & Thermodynamics Level: High School
Calorimetry
Thirty grams of ice , initially at -10 degreeC , are put into 300 gms of water , originally at 75 degree C , contained in a glass beaker . The final temperature of the system is 64.5 degreeC . Ignore any heat loss from the glass beaker . Find the heat of fusion of the ice .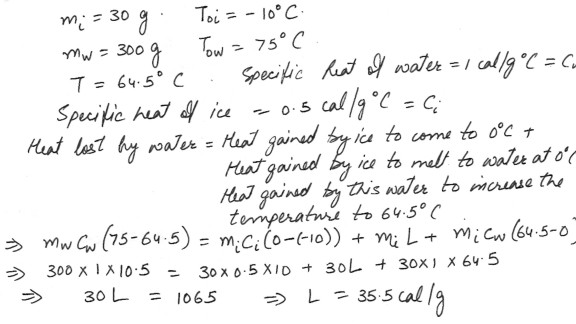
Physics Heat & Thermodynamics Level: High School
7Density can be defined as molar mass, MM, divided by molar volume , V .(a) determine (derivative of d / derivative of T) for an ideal gas in terms of MM , V , and p
Interpret your response to (a), especially with respect to the sign expected for the values of the derivative .

Physics Heat & Thermodynamics Level: High School
A desperate chem 321 student needed a sample of a pure gas for a series of experiments on gas expansions. But alas no tank of pure gases were available , undaunted , he decided to use Br2, and to work at temperatures above its boiling point. She first obtained Br2 vapor to fill a 5.000 L glass bulb at 150.0 degrees Celsius and 1.00 atm, the performed the following experiments.Expansion of final volume of 10.000 L against a constant pressure of .300 atm, holding the temperature constant, Calculate change in U and H, w, and q.
Reversible, isothermal, expansion to a final volume of 10.000L. calculate change in E and H, w,q